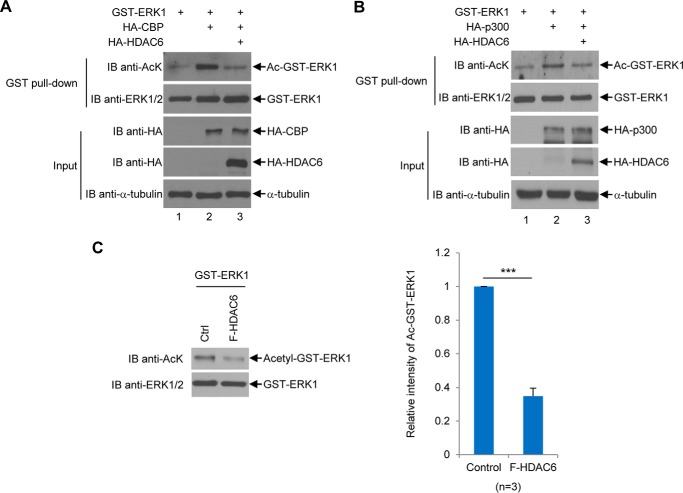
Figure 9.
HDAC6 deacetylates ERK1 in vivo and in vitro. A, HDAC6 decreases CBP-induced ERK1 acetylation in vivo. GST-ERK1 was transfected into HEK293T cells alone, with CBP, or with CBP and HDAC6. GST-ERK1 proteins were purified as described under the “Experimental procedures” followed by the anti-AcK Western blot analysis. The membrane was then stripped and then reprobed with anti-ERK1/2 antibodies. The input was subjected to Western blot analyses with indicated antibodies. B, HDAC6 decreases p300-induced ERK1 acetylation in vivo. The experiments were performed as described in A, except that HA-p300 was used to replace HA-CBP. C, HDAC6 deacetylates ERK1 in vitro. Left panels, acetylated bacterially purified GST-ERK1 protein was incubated with or without F-HDAC6 purified from 293T cells as described under the “Experimental procedures” followed by the anti-AcK Western blot analysis. The procedure for generating acetylated GST-ERK1 was described under the “Experimental procedures.” The membrane was stripped and reblotted with the anti-ERK1/2 antibody. Right panel, relative intensity of acetylated GST-ERK1 band was quantified by densitometry against the total ERK1 band shown in a bar graph. This experiment was repeated three times. Student's t tests were performed with ***, p < 0.001. Error bars, S.D. IB, immunoblot.