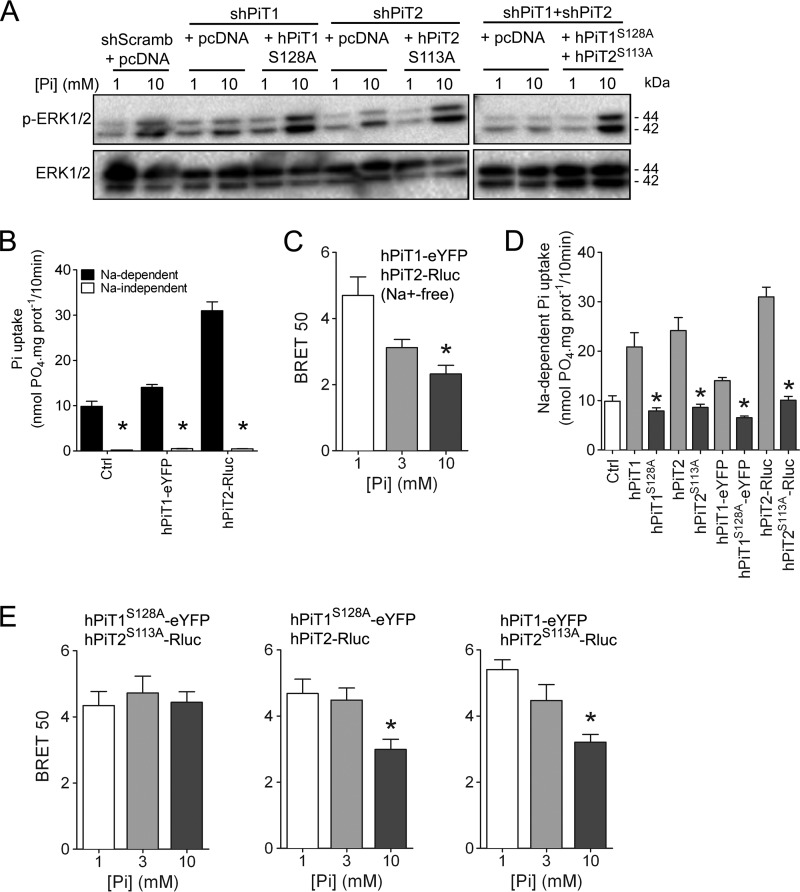
Figure 4.
Pi-dependent ERK1/2 signaling and PiT1-PiT2 hetero-oligomerization are independent of Pi transport. A, Western blot analysis of ERK1/2 phosphorylation (P-ERK 1/2) in transiently transfected MC615 cells, as indicated. Overexpression of Pi transport–deficient hPiT1 (PiT1S128A) and/or hPiT2 (PiT2S113A) was performed in PiT1–, PiT2–, or PiT1-PiT2–depleted cells, respectively. Cells were incubated in low-serum (0.5%) medium for 24 h and stimulated for 30 min with 1 or 10 mm extracellular Pi concentration. Total ERK1/2 proteins were used as a loading control. B, sodium-dependent and -independent Pi uptake was measured in HEK293T cells transfected as indicated. Data are means ± S.E. (error bars) (*, p < 0.05 versus sodium-dependent, n = 4). C, BRET 50 index was measured from BRET saturation curves obtained after a 10-min stimulation with 1, 3, or 10 mm extracellular Pi concentration from HEK293T co-transfected with hPiT1-eYFP and hPiT2-Rluc in an Na+-free medium. Data are means ± S.E. (*, p < 0.05 versus 1 mm Pi condition, n = 4). D, sodium-dependent Pi uptake was measured in HEK293T cells transfected with pcDNA6A plasmid (Ctrl) or with plasmids containing normal (hPiT1 and hPiT2) or Pi transport–deficient mutants (hPiT1S128A and hPiT2S113A) with or without BRET chimeric acceptor or donor, as indicated. Data are means ± S.E. (*, p < 0.05 versus Pi-transporting PiTs, n = 4). E, BRET 50 index was measured from BRET saturation curves obtained after 1, 3, or 10 mm extracellular Pi concentration stimulation for 10 min from HEK293T co-transfected with the indicated plasmids. Data are means ± S.E. (*, p < 0.05 versus 1 mm Pi condition, n = 4–5).