Figure 1.
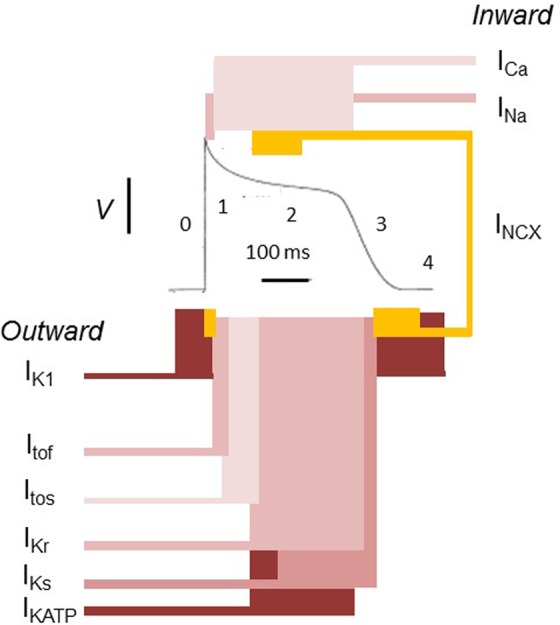
The ventricular action potential as a paradigm for cardiac electrophysiological activity. In the resting state, the voltage of the cell intracellular space is negative to the external environment. This reflects its higher K+ but lower Na+ and Ca2+ concentrations and its lower membrane permeability to Na+ and Ca2+ in comparison to K+. K+ efflux from the cell is then controlled by the inward rectifier K+ channel (IK1). When excitation threshold is reached, a large Na+ influx (INa) into the cell through Na+ channels produces phase 0 depolarization. This is followed by activation of fast and slow transient outward K+ currents (Itof and Itos, respectively) mediating a K+ efflux driving a rapid phase 1 repolarization. There is also an activation of a depolarizing inward Ca2+ current through L-type Ca2+ channels (ICa), which initiates excitation contraction coupling. The reduced membrane K+ permeability due to IK1 rectification combined with ICa maintains the action potential phase 2 plateau phase. Phase 3 repolarization is driven by K+ efflux through the rapid and slow delayed rectifier K+ channels (IKr and IKs, respectively), as well as IK1. At the end of phase 3, the Na+ and Ca2+ that have accumulated in the cells are removed by the Na+, K+ pump, and the Na+, Ca2+ exchanger (NCX). The atrial action potential shows greater contributions to recovery from the ultrarapid delayed rectifier outward currents (IKur) and acetylcholine-activated inward rectifying K+ channel (IKACH). Adapted with permission from Huang.1
