Abstract
Background
Testosterone levels and physical activity (PA) each play important roles in men’s health, but the relationship between the two remains unclear.
Methods
We evaluated the cross-sectional association between self-reported total PA and serum testosterone levels in 738 men (mean age 42.4 yrs, range 20-≥85yrs) who participated in NHANES 1999–2004. We compared geometric mean testosterone concentrations measured by RIA and calculated the odds ratio (OR) of having low or low normal testosterone (≤3.46 ng/mL) across tertiles of total PA in all men, and men stratified by age (20–49, ≥ 50 years), and obesity status (BMI <30, ≥ 30 kg/m2).
Results
The geometric mean testosterone concentration was 5.31 ng/mL; 18.6% of the men had low or low normal serum testosterone levels. PA tertiles were not associated with testosterone levels overall, or when stratified by age or obesity status. Similarly, there was no association between PA tertiles and the odds of low or low normal testosterone, overall or by age. However, among non-obese men, those in the highest PA tertile were significantly less likely to have low or low normal testosterone than those in the lowest tertile (OR 0.50; 95% CI = 0.26–0.95); there was no association among obese men.
Conclusions
Greater PA was not associated with testosterone levels, but may be associated with a reduced odds of low or low normal testosterone in non-obese men, but not in obese men.
Keywords: testosterone, hormones, physical activity
Background and Significance
Sex steroid hormone levels play an important role in men’s health (Shiels et al. 2009; Gooren et al. 2007). Specifically, testosterone affects the growth and maturation of the prostate, and other male sex organs, the development of male hair distribution, changes in body muscle mass and fat distribution, bone and muscular strength, sex drive and sexual function, mood, and energy level (Gooren et al. 2007; Ohl and Quallich 2006). Testosterone levels are known to decrease with age and obesity (Harman et al. 2001; Feldman et al. 2002; Svartberg et al. 2003; Yeap 2009; McHenry 2012; Gruenewald and Matsumoto 2003), but increase with cigarette smoking (Shiels et al. 2009). In men, low levels of sex steroid hormones have been associated with an increased risk of prostate cancer (Platz and Giovannucci 2004), heart disease (Khaw et al. 2007; Abbott et al. 2007; Khaw and Barrett-Connor 1991; Tivesten et al. 2006), diabetes (Selvin et al. 2007), low bone mineral density (Kuchuk et al. 2007), and symptoms of depression and fatigue (McHenry 2012). While there is no universally accepted threshold for clinically low testosterone (Paduch et al. 2014), men suffering from low testosterone levels (≤ 12nmol or 3.46 ng/mL) may be offered testosterone replacement therapy (Wang et al. 2009). However, knowledge of the long-term risks and outcomes of testosterone replacement therapy is still limited (Gruenewald and Matsumoto 2003; Nigro and Christ-Crain 2012; Surampudi, Wang, and Swerdloff 2012). Given the importance of androgens in the general health of adult men, the increasing number of aging and obese men, and the uncertainty of the risks and benefits of testosterone therapy, identifying modifiable factors that influence hormone levels, especially those that enhance overall health, has become an important issue in men’s health (Wang et al. 2009).
Physical activity is beneficial for men’s health overall, and may also influence testosterone levels (Petersen and Pedersen 2005; Müller-Riemenschneider et al. 2010). At present, the relationship between physical activity levels and male sex steroid hormone levels remains unclear. Some cross-sectional studies have found greater amounts of habitual physical activity to be associated with higher testosterone levels (Shiels et al. 2009; Muller et al. 2003; Ari et al. 2004; Allen et al. 2002; Goh et al. 2007; Mantzoros and Georgiadis 1995; Yeap et al. 2009), while others show no association between physical activity and testosterone levels (Allen et al. 2002; Handa et al. 1997; Lindholm et al. 1982; Dai et al. 1981; Ponholzer et al. 2005; Camacho et al. 2013). Physical activity intervention studies have also yielded inconsistent findings regarding the relationship between physical activity and testosterone levels (White et al. 2002; Hall et al. 1999; Hawkins et al. 2008; Heufelder et al. 2009). The few studies that have specifically explored the association between physical activity and low or low normal testosterone have observed an inverse association (Tajar et al. 2010; Yeap et al. 2009)
Thus, we evaluated the association between self-reported total physical activity and testosterone level and low or low normal level in a cross-sectional study among a nationally representative sample of non-institutionalized men using the most recent hormone data from NHANES (1999–2004). This study used an improved assessment of total physical activity (domestic, transportation, and leisure) compared with NHANES III (1988–1994), which only included frequency of moderate and vigorous activities across domains (Centers for Disease Control and Prevention 2012d). Starting in 1999, NHANES updated the physical activity questions to capture duration and intensity of activities, which provides a more precise estimate of physical activity.
Methods
Study Population
The National Health and Nutrition Examination Survey (NHANES) is a continuous cross-sectional survey conducted by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention. Using complex, multi-stage sampling, it was designed to assess the health and nutritional status of non-institutionalized citizens of the United States of ages 2 months and older (Centers for Disease Control and Prevention 2012a). A detailed description of the data collection methodology of the NHANES has been previously published (Centers for Disease Control and Prevention 2012a). To produce more precise estimates for certain population subgroups, children, older adults, non-Hispanic blacks, and Mexican-Americans were oversampled. All participants were interviewed and physical examinations were performed at a mobile examination center.
NCHS releases public-use data files in 2-year cycles and unbiased national estimates can be independently produced for each 2-year cycle. Concatenated data files can be created to produce multi-year samples due to standardized methodology and measures used to collect the data (Centers for Disease Control and Prevention 2012a). The NCHS Research Ethics Review Board approved all protocols and each participant gave informed consent (Centers for Disease Control and Prevention 2012a). This analysis utilized data from the interview and examination components of NHANES 1999–2004. Testosterone concentration was measured in stored serum samples in males 12 years and older who participated in the morning examination session (n =1,517) (Centers for Disease Control and Prevention 2012c). This analysis included men who were 20 years or older and had available serum samples in the repository (n=990). We excluded men if they reported a history of prostate cancer (n=15), reported limitations to engaging in physical activity (i.e. unable to walk without an assistive device (n=57)), or were missing information on physical activity or other covariates of interest (i.e. age, race, body mass index (BMI), alcohol consumption, smoking status, and serum cotinine (n=180). The final sample size for this analysis was 738 men.
Assessment of physical activity
Physical activity was assessed by interview using a questionnaire (Centers for Disease Control and Prevention 2012d). Men reported their participation in three domains of physical activity: daily transportation, domestic and leisure-time activities. Participants identified the number of times during the past 30 days that they walked or bicycled for transportation, participated in moderate-intensity activities while around the home, and participated in moderate- or vigorous-intensity activities during their leisure-time, including exercise, sports, or active hobbies (jogging, running, bicycling, swimming, dance, golf, gardening, yard work, weight lifting etc.) (Centers for Disease Control and Prevention 2012b). Duration was quantified for each activity, and activities lasting less than 10 minutes were excluded from analysis (Centers for Disease Control and Prevention 2012b). Each activity was assigned a metabolic equivalent of task (MET) score based on the compendium of physical activities (Ainsworth et al. 2000). METs are a unit used to describe the energy expenditure of a specific activity, where one MET = 3.5 ml/kg/min. We calculated MET-minutes per week of total physical activity, which included the combined total METs of daily transportation, domestic and leisure-time activities. We categorized total physical activity into tertiles (Tertile 1: ≤ 499 MET-minutes per week, or ≤ 2.5 weekly hours/≤ 20 daily minutes of moderate-intensity physical activity; Tertile 2: 500 – 1554 MET-minutes per week, or > 2.5 to 7.5 weekly hours/> 20 to 65 daily minutes of moderate-intensity physical activity; Tertile 3: > 1554 MET-minutes per week, or > 7.5 weekly hours/> 65 daily minutes of moderate-intensity physical activity). The most recent Physical Activity Guidelines for Americans recommends 500 MET-minutes per week for the promotion and maintenance of health in adults, however more physical activity may be associated with additional health benefits (U. S. Department of Health and Human Services 2008).
Assessment of Additional Covariates
During the interview component of NHANES, demographic and health behavior data were collected including: age (NHANES codes all participants aged 85 and older at examination as age 85 in these public use data; n=8), race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, and other), cigarette smoking (never, former, and current), and alcohol consumption (the sum of monthly beer, wine, and hard liquor intake). We categorized the frequency of total alcohol consumption as 0 drinks per day, < 2 drinks/day, ≥ 2 drinks/day. We used the Dietary Guidelines for Americans definition of moderate drinking for men of no more than 2 drinks per day(U. S. Department of Health and Human Services 2010), but placed non-drinkers into their own category because they tend to be very different on many characteristics than people who do not drink any alcohol. Trained health technicians took body measures including: height, weight, and waist circumference during the examination. BMI was calculated using the formula: body mass (kg) / [height (m)]2. Exposure to environmental tobacco smoke was obtained through the measurement of serum cotinine, a metabolite of nicotine, captures both passive and active exposures as well as the actual amount smoked. We categorized cotinine level as unexposed: < 0.35 ng/mL, passively exposed: 0.35 - 9.99 ng/mL, and actively exposed: ≥ 10 ng/mL (Bernert et al. 1997).
Measurement of serum testosterone concentration
Following an overnight fast, men’s blood samples were drawn between 8:30 and 11:30am at the medical examination center or during an abbreviated examination at home (Centers for Disease Control and Prevention 2012c). Surplus specimens were separated into their components and were stored at −70°C (Centers for Disease Control and Prevention 2012c). Specimens were assayed for sex steroid hormones at Boston Children’s Hospital (Boston, MA) by laboratory technicians who were blinded to participant characteristics. Serum testosterone concentrations were quantified using a competitive electrochemiluminescence immunoassay on the 2010 Elecsys autoanalyzer (Roche Diagnostics, Indianapolis, IN). The lowest detection limit of the assay was 0.02 ng/mL (Centers for Disease Control and Prevention 2012c). The coefficient of variation (CVs) for testosterone was 4.8% (Centers for Disease Control and Prevention 2012c). Men were categorized as having low or low normal testosterone if their serum testosterone concentration was ≤ 3.46 ng/mL. This cut point corresponds to the total testosterone concentration at which men are recommended to consider hormone replacement therapy by the International Society of Andrology, the International Society for the Study of Aging Male, the European Association of Urology, the European Academy of Andrology (EAA) and the American Society of Andrology (Wang et al. 2009). Because we are specifically interested in identifying potentially modifiable factors that may provide an alternative to hormone replacement therapy, in sub-analyses, we also categorized men as having low normal testosterone if their serum testosterone concentration was ≤ 3.46 ng/mL and > 2.31 ng/ml. In these analyses, we excluded men with serum testosterone concentration ≤ 2.32 ng/mL (n=40); this cutpoint corresponds to the total testosterone level at which men are recommended to take hormone replacement therapy (Wang et al 2009), and thus they would not be targeted for alternative strategies.
Minimal Detectable Associations
This study used existing public-use data, so the sample size was fixed. Thus, we present minimal detectable differences for the primary analyses, rather than sample size calculations, for our analysis of the association between physical activity and low or low normal testosterone. For the total sample size of 738 men, among whom 142 had low testosterone, for a power of 80%, and alpha=0.05, and a two-sided test, we could detect as statistically significant an OR of 0.49 or lower in the top compared with the bottom tertile of physical activity. This sized association would be of clinical importance. Among the non-obese men, for the sample size of 538 men, among whom 67 had low testosterone, for a power of 80%, and alpha=0.05, and a two-sided test, we could detect as statistically significant an OR of 0.36 or lower in the top compared with the bottom tertile. Among the obese men, for the sample size of 200 men, among whom 75 had low testosterone, for a power of 80%, and alpha=0.05, and a two-sided test, we could detect as statistically significant an OR of 0.33 or lower in the top compared with the bottom tertile.
Statistical Analysis
Following the recommended guidelines from the NCHS (Centers for Disease Control and Prevention 2012a), a 6-year sampling weight was used for all analyses to account for the complex NHANES sampling design, including unequal probabilities of selection, over-sampling, and non-response. Age and race adjusted geometric means and proportions and their 95% confidence intervals (CIs) for demographic and other factors were calculated using linear regression models. Multivariable linear regression was used to estimate and compare geometric mean testosterone concentrations across physical activity tertiles. Tests for trend were conducted across all levels of physical activity using the Wald chi-square test. We also modeled continuous physical activity using restricted quadratic splines with knots at the 10th, 50th, and 90th percentiles; we excluded the top and bottom 1% of the distribution of physical activity and testosterone from the restricted quadratic splines model to avoid the influence of extreme values. Logistic regression was used to estimate the odds ratio (OR) and 95% confidence interval (95% CI) of low or low normal testosterone (≤ 3.46 ng/mL) comparing the middle and top tertiles of physical activity to the lowest tertile. In sub-analyses, logistic regression was used to estimate the odds ratio (OR) and 95% confidence interval (95% CI) of low normal testosterone (2.31 ng/mL ≤ testosterone ≤ 3.46 ng/mL) comparing the middle and top tertiles of physical activity to the lowest tertile; men with serum testosterone ≤ 2.31 ng/mL (n=40) were excluded from these analyses.
All models were adjusted for age, race/ethnicity, smoking status, cotinine as a measure of exposure to environmental tobacco smoke, alcohol consumption, BMI, and waist circumference. Additionally, analyses were stratified by age category (20–49, ≥ 50 years), and obesity status (BMI < 30, ≥ 30 kg/m2). We also tested for interactions by age category and obesity status by including their cross-product terms in a separate model. For all tests the p-values were two sided with a = 0.05. Statistical analyses were performed using SAS v.9.2 (Cary, NC), SAS-callable SUDAAN, v10.0 (RTI International, Research Triangle Park, NC, USA), and R version 3.2.0 software.
Results
In the total sample, the geometric mean testosterone concentration was 5.31 ng/mL, with 18.6% of the men having low or low normal serum testosterone levels. The proportion of men of various races/ethnicities significantly varied across tertile of physical activity, with a greater proportion of non-Hispanic black and Mexican-American men in the least active tertile (Table 1). The least active men were also more likely to report former or current cigarette smoking than were more active men, though this difference was not statistically significant. In all activity tertiles, mean BMI of the men fell within the overweight category for adults (Ogden et al. 2006). There were no other statistically significant differences in the characteristics of men by physical activity tertile.
Table 1.
Age and race adjusted characteristics of 738 men 20 years or older who participated in NHANES 1999–2004 by tertile of weekly physical activity.a
| ≤ 499 METsb (N = 277) |
500–1554 METs (N = 226) |
≥ 1555 METs (N = 235) |
p-value | |
|---|---|---|---|---|
|
|
||||
| Mean or percentage (SE)c | ||||
| Aged (years) | 41.0 (1.1) | 44.0 (0.9) | 42.1 (1.1) | 0.13 |
| Race/ethnicitye (%) | <0.01 | |||
| Non-Hispanic white | 64.0 (4.0) | 74.0 (4.0) | 77.0 (3.0) | |
| Non-Hispanic black | 13.0 (2.0) | 9.0 (1.0) | 9.0 (2.0) | |
| Mexican-American | 13.0 (2.0) | 7.0 (1.0) | 5.0 (1.0) | |
| Other | 10.0 (3.0) | 11.0 (3.0) | 9.0 (2.0) | |
| Body mass index (kg/m2) | 28.2 (0.7) | 27.9 (0.4) | 27.5 (0.4) | 0.58 |
| Waist circumference (cm) | 100.5 (1.5) | 99.4 (1.0) | 97.0 (0.9) | 0.10 |
| Cigarette Smoking (%) | 0.07 | |||
| Never | 36.0 (4.0) | 51.0 (4.0) | 51.0 (4.0) | |
| Former | 31.0 (4.0) | 21.0 (3.0) | 24.0 (3.0) | |
| Current | 32.0 (3.0) | 28.0 (3.0) | 25.0 (4.0) | |
| Urinary cotinine (%)f | 0.07 | |||
| Unexposed | 50.0 (3.0) | 54.0 (4.0) | 50.0 (4.0) | |
| Passive exposure | 14.0 (2.0) | 8.0 (2.0) | 17.0 (2.0) | |
| Active exposure | 37.0 (3.0) | 38.0 (4.0) | 33.0 (4.0) | |
| Alcohol consumption (%) | 0.94 | |||
| 0 drinks/month | 21.0 (3.0) | 19.0 (4.0) | 17.0 (4.0) | |
| < 2 drinks/day | 68.0 (3.0) | 69.0 (4.0) | 70.0 (3.0) | |
| ≥ 2 drinks/day | 11.0 (2.0) | 12.0 (3.0) | 13.0 (2.0) | |
Sampling weights were applied. Means and proportions were adjusted for age, race/ethnicity;
MET: metabolic equivalent (MET)-minutes per week;
SE: standard error;
Race adjusted;
Age adjusted;
A measure of environmental tobacco smoke exposure; Unexposed: < 0.35 ng/mL; Passive exposure: ≥0.35 - <10.0 ng/mL; Active exposure: ≥10 ng/mL
Total Testosterone Concentration
Mean testosterone concentration was not significantly different across tertiles of physical activity overall or when stratified by age or BMI (Table 2). Men in the highest tertile of physical activity appeared to have higher concentrations of testosterone than men in the lowest and middle tertiles of physical activity, although the differences were not statistically significant (Table 2). In stratified analyses, the trend of non-significantly higher concentrations of mean testosterone among men in the highest tertile of activity was consistent within the 20–49 years age category, regardless of obesity status. Although not significant, in general, younger men had higher concentrations of testosterone than older men, and non-obese men had higher concentrations of testosterone than obese men across all tertiles of physical activity. We observed similar patterns, overall and within age and BMI strata, when physical activity was modeled as continuous variable (Supplemental Figure 1).
Table 2.
Multivariable adjusted geometric mean (95% CI) testosterone concentration by tertile of weekly physical activity in men 20 years or older, NHANES (1999–2004).
| n | ≤ 499 METsa | 500–1554 METs | ≥1555 METs | p-trend | |
|---|---|---|---|---|---|
|
|
|||||
| Total | 738 | 5.26 (4.95–5.57) | 5.00 (4.75–5.25) | 5.65 (4.83–6.47) | 0.26 |
| Age (yrs) | |||||
| 20 -< 50 | 447 | 5.61 (5.26–5.96) | 5.21 (4.86–5.56) | 6.17 (4.95–7.39) | 0.23 |
| ≥ 50 | 291 | 4.51 (4.10–4.92) | 4.50 (4.21–4.79) | 4.53 (4.22–4.84) | 0.99 |
| BMI (kg/m2) | |||||
| < 30 | 538 | 5.62 (5.29–5.95) | 5.36 (4.99–5.73) | 5.71 (5.46–5.96) | 0.30 |
| ≥ 30 | 200 | 4.34 (3.81–4.87) | 4.11 (3.68–4.54) | 5.47 (2.88–8.06) | 0.64 |
MET: metabolic equivalent (MET)-minutes per week. Within the same physical activity tertile we cannot statistically compare the age and BMI categories to one another because the estimates are generated using separate statistical models. All models were adjusted for age, race/ethnicity, smoking, cotinine, alcohol consumption, body mass index (BMI), and waist circumference.
Low or Low Normal Testosterone Concentration
Physical activity tertiles were not associated with low or low normal testosterone concentrations overall, or when stratified by age. Overall, men in the highest tertile of physical activity (OR 0.84; 95% CI = 0.52–1.35), and men in the middle tertile (OR 0.99; 95% CI = 0.56–1.75, p for trend = 0.72) did not have lower odds of low or low normal testosterone compared to men in the lowest tertile of physical activity after adjustment for all covariates. In younger men (20–49 years), tertile of physical activity was not associated with low or low normal testosterone (OR, highest to lowest tertile 0.89; 95% CI = 0.43–1.82, middle to lowest tertile 1.07; 95% CI = 0.50–2.29, p for trend = 0.87). Similarly, in older men (≥ 50 years), tertile of physical activity was not associated with low or low normal testosterone (OR, highest to lowest tertile 0.82; 95% CI = 0.36–1.88, middle to lowest tertile 1.02; 95% CI = 0.45–2.33, p for trend = 0.83) after adjusting for all covariates.
When men with low testosterone were excluded in sub-analyses, physical activity tertiles were suggestive of a lower odds of low normal testosterone concentrations overall, and when stratified by age, though these associations were not statistically significant. Overall, men in the highest tertile of physical activity (OR 0.79; 95% CI = 0.38–1.64), and men in the middle tertile (OR 0.70; 95% CI = 0.37–1.34, p for trend = 0.53) had non-statistically significant lower odds of low normal testosterone compared to men in the lowest tertile of physical activity. In younger men (20–49 years), men in the highest tertile of physical activity (OR 0.62; 95% CI = 0.27–1.39) had non-statistically significant lower odds of low normal testosterone compared to men in the lowest tertile of physical activity; men in the middle tertile did not have lower odds of low normal testosterone compared to men in the lowest tertile of physical activity (OR 0.93; 95% CI = 0.39–2.19, p for trend = 0.37). In older men (≥ 50 years), tertile of physical activity was not statistically significantly associated with low normal testosterone (OR, highest to lowest tertile 0.79; 95% CI = 0.29–2.12, middle to lowest tertile 0.71; 95% CI = 0.24–2.04, p for trend = 0.79).
The prevalence of obesity (BMI ≥30 kg/m2) in the participants was 27.0%. Among non-obese men, those in the highest tertile of physical activity were 50% less likely (95% CI: 0.27–0.96, p=0.02) to have low or low normal testosterone than men in the lowest tertile (Figure 1). Subanalysis results were similar excluding men with low testosterone: among non-obese men, those in the highest tertile of physical activity were significantly less likely (OR 0.35; 95% CI: 0.14–0.89) to have low normal testosterone than men in the lowest tertile and there was a possible trend across tertiles (middle tertile: OR 0.81 95% CI: 0.31–2.12, p for trend = 0.07). Among obese men, there was no association between physical activity level and low or low normal testosterone (Figure 1). Subanalysis results were similar excluding men with low testosterone: among obese men, there was no association between physical activity level and low normal testosterone (OR, highest to lowest tertile 1.81, 95% CI: 0.53–2.63, middle to lowest tertile 1.00, 95% CI: 0.26–3.90, p for trend = 0.47). There was no significant interaction between BMI category (p interaction = 0.21) and physical activity tertile in the analysis of low and low normal testosterone. When the analysis of low and low normal testosterone was restricted to men ≥50 years, the inferences when stratified by BMI category were the same (data not shown).
Figure 1.
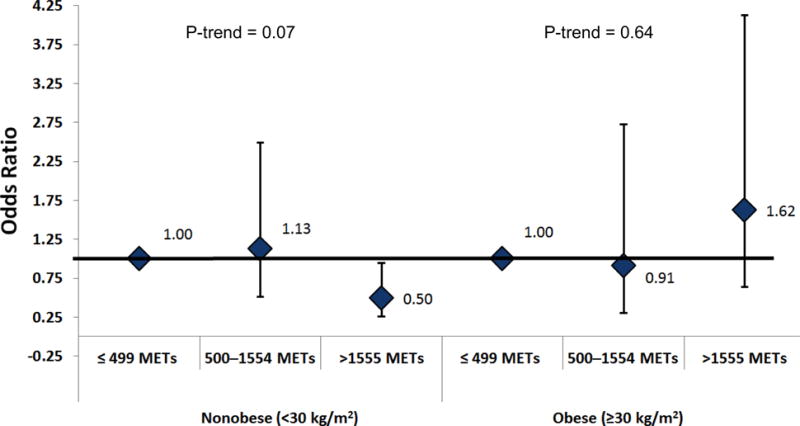
Odds ratio of low or low normal testosterone by physical activity tertiles in nonobese (n=538) and obese (n=200) men 20 years or older; NHANES (1999–2004).
MET: metabolic equivalent (MET)-minutes per week. Models were stratified by the body mass index cut point for obesity (<30 kg/m2 and ≥30 kg/m2). All models were adjusted for age, race/ethnicity, smoking, cotinine, alcohol consumption, BMI, and waist circumference; ≤ 499 METs = reference group. P for interaction = 0.21
Discussion
In this cross-sectional analysis of a nationally representative sample of adult men, self-reported physical activity was not significantly associated with testosterone concentration in men overall, or by age or obesity status. We did observe a pattern in which the most active men had non-significantly higher mean testosterone concentrations than did less active men; this pattern was present among younger men and obese men. There was no association between physical activity level and low or low normal testosterone overall, or by age. However, among non-obese men, increased physical activity was associated with a significant decrease in the odds of low or low normal testosterone. Specifically, men who reported more than 65 minutes of physical activity daily had half the risk of low or low normal testosterone compared to men who reported 25 minutes or less of physical activity daily. Because our study was cross-sectional, however, we cannot distinguish the temporal direction of the association between physical activity and serum testosterone concentration.
Our observation that more active, non-obese men appeared to have higher testosterone concentrations is consistent with several previous cross-sectional studies. In the only other population-based US study, our group previously reported that men (N=1275) 20-≥85 years old participating in NHANES III (1988–1994) who were in the highest quartile of total physical activity had significantly higher concentrations of total testosterone compared to those who reported no physical activity (Shiels et al. 2009). Our earlier study used a less detailed physical activity questionnaire than the current study, and it only allowed for the determination of frequency (times per week) of physical activity, and not duration or intensity. In a Dutch cross-sectional study of 400 middle-and old-aged men (40–80 years), higher testosterone levels were observed in men in the highest quartile of physical activity (Muller et al. 2003). Men’s physical activity during the past year was scored and classified into categories ranging from low (< 4500 kJ/day) to high (≥ 6000 kJ/day) using cutoffs that are comparable to the ones used in our study. In the European Prospective Investigation into Cancer and Nutrition (EPIC), a cross-sectional study consisting of 696 men 20+ years old, Allen et al. observed significantly higher testosterone concentrations in men who reported engaging in vigorous physical activity for three or more hours per week compared to men who reported zero hours per week (Allen et al. 2002). However, no association was observed between total recreational exercise-time (< 7.5, 7.5–14, or >14 hrs/week) and testosterone concentrations (Allen et al. 2002). The upper end of the lowest category of physical activity was equivalent to the highest category of physical activity in our current and other previous studies (Shiels et al. 2009; Muller et al. 2003). The lack of association between total recreational exercise-time and testosterone concentrations may be a result of the large variation in their categorization of exercise–time, or due to the potential for their lowest category reference group to have high levels of physical activity. Our study and others have shown that the association between physical activity and testosterone concentration is generally evident when comparing those who do the least amount of activity to those who the most (Muller et al. 2003; Shiels et al. 2009). The extent of obesity was much higher in our study (27%) than in previous studies (17%) (Shiels et al. 2009). The prevalence of obesity was even lower in the European studies (12% in the Dutch study (Muller et al. 2003), 6% in the EPIC study) (Allen et al. 2002). Despite the differences in physical activity questionnaires, physical activity profiles, ages, obesity status, and countries of the study populations, the positive associations between physical activity and testosterone appeared to be consistent across these studies. In general, men who engaged in more exercise had higher testosterone levels than men who did not.
We did not observe an association between physical activity and low or low normal testosterone in men overall. Our findings were suggestive, though not statistically significant, of a possible inverse association between physical activity and low normal testosterone. Prior studies on physical activity and low testosterone have reported inverse associations. In a cross-sectional analysis of men 40–79 years in the European Male Aging Study, physical activity was significantly associated with late onset hypergonadism after adjustment for age, BMI, smoking status and comorbidities (Tajar et al. 2010). A follow-up analysis of men in this same study (mean follow-up 4.4 yrs) reported no statistically significant influence of physical activity change on testosterone levels, but men who reported increased activity between baseline and follow-up had a non-significant lower decrease in testosterone than men who maintained or reduced activity (Camacho et al. 2013). Similarly, men who were 65 and over and participated in ≥3hrs of activity weekly were less likely to be in the lowest quartile of testosterone in the Men in Health Study (Yeap et al. 2009). Unlike these studies, our study included men as young as 20 years old. The biologic mechanisms underlying low testosterone in younger men may different than in older men, thus, the influence of modifiable risk factors on low testosterone may also be different.
In our study, non-obese men in the highest tertile of physical activity had the lowest likelihood of having low or low normal testosterone in comparison to non-obese men in the lowest tertile of physical activity. Low or low normal testosterone has been associated with many adverse health outcomes including obesity, metabolic syndrome and type 2 diabetes (Stanworth and Jones 2009; Wang et al. 2011), higher grade prostate cancer (Platz and Giovannucci 2004), heart disease (Khaw et al. 2007; Abbott et al. 2007; Khaw and Barrett-Connor 1991; Tivesten et al. 2006), low bone mineral density (Kuchuk et al. 2007), depression and fatigue (McHenry 2012). The symptoms associated with low testosterone levels affect more than 4.5 million men in the US (McHenry 2012), and much is still unknown regarding the long-term risks and outcomes of testosterone replacement therapy (Gruenewald and Matsumoto 2003; Nigro and Christ-Crain 2012). Physical activity, which has many health benefits (Petersen and Pedersen 2005; Müller-Riemenschneider et al. 2010), may help maintain normal testosterone levels in aging men through the control of obesity and diabetes (Liu et al. 2009), though few studies have evaluated this association. Similarly, a 52-week intervention trial in men with clinically low testosterone found that improving diet and increasing physical activity raised serum testosterone concentrations to the lower range of normal for the 16 men in the supervised diet and exercise intervention group (Heufelder et al. 2009). In our study, the risk of low or low normal testosterone was restricted to non-obese men; this may suggest that even high levels of physical activity may not be enough to overcome the adverse influence of extensive adiposity on testosterone levels, or that there is a greater misclassification of self-reported physical activity among obese men than among lean men.
The study had both strengths and limitations. This cross-sectional study was based on a nationally representative sample of U.S. men in which physical activity and testosterone measures were collected at the same point in time. Thus, causality cannot be inferred. The sample size was fixed. In general, the study was powered to detect moderate or larger associations, which would be most likely to be of clinical relevance. The updated NHANES physical activity questionnaire captured both duration and intensity of activities, across several domains of physical activity (domestic, transportation, and leisure), which permitted a more precise estimation of physical activity. We were unable to use objective measures of physical activity, because accelerometry was only available on 23% of the analytic sample. All analyses were adjusted for important confounders that we previously found to be associated with testosterone concentration in NHANES III, including both self-reported cigarette smoking and urinary cotinine levels (Shiels et al., 2009) and two measures of adiposity, BMI and waist circumference (Rohrmann et al., 2011), collected by trained study staff. Study limitations included that the analysis was based on a single measure of testosterone, however, all samples were collected in the morning session, which is likely to reduce differences due to diurnal variation. In addition, the study was unable to account for occupational activity as the data were not collected.
Physical activity is recognized as an important factor influencing health and disease status. The benefits of physical activity include improved cardiovascular fitness; decreased coronary heart disease and diabetes; the preservation of fat-free mass and bone mineral density; and improved mood and self-esteem (Ballor and Poehlman 1994; Blair 2009; American College of Sports Medicine 1998). Conversely these physiological changes are negatively affected by low levels of testosterone (American College of Sports Medicine 1998; Ballor and Poehlman 1994; Blair 2009). While the role of physical activity in the relationship between obesity and testosterone is unclear, our results suggest that greater physical activity may reduce the likelihood of low or low normal testosterone concentrations in non-obese men, but not in obese men. We could not, however, distinguish between physical activity influencing serum testosterone concentration and testosterone concentration influencing physical activity in this cross-sectional analysis. Previous research supports the relationship between lower levels of testosterone and lower levels of strength and muscle mass in aging men (Srinivas-Shankar and Wu 2009; Breuer et al. 2001), which may be predictive of engagement in physical activity, and activities of daily living. Thus, studies are needed to determine whether greater activity would prevent men from developing low testosterone and whether men who already have low or low normal testosterone would benefit from increasing physical activity.
Supplementary Material
Supplemental Figure 1 Legend: Multivariable-adjusted association between physical activity and testosterone using restricted quadratic splines with knots at the 10th, 50th, and 90th percentiles. The top and bottom 1% of the distribution of physical activity and testosterone were excluded from the restricted quadratic splines model to avoid the influence of extreme values. Models were adjusted for age, race/ethnicity, smoking status, cotinine as a measure of exposure to environmental tobacco smoke, alcohol consumption, BMI, and waist circumference. Gray shading: Represents confidence intervals. Background: Distribution of physical activity in study population. A: All men. B: Men, 20–49 years. C: Men, ≥ 50 years. D: Non-obese men, BMI < 30 kg/m2. E: Obese men, BMI ≥ 30 kg/m2.
Acknowledgments
Funding/Support: Steeves, J. A. was supported as a Cancer Prevention Fellow by the National Cancer Institute. Joshu, C.E. was supported by grants from the Seraph Foundation and the Prostate Cancer Foundation. Support for the hormone assays was provided by the National Institutes of Health Intramural Research Program to McGlynn, K.A.
Role of the Sponsor: The funders played no role in the design or conduct of this research
Footnotes
Author Contributions:
Study concept and design: Steeves, J. A., Joshu, C. E., Platz, E. A.
Acquisition of data: Bradwin, G., McGlynn, K. A.
Analysis and interpretation of data: Steeves, J. A., Peskoe, S., Fairchild, V., Joshu, C. E., Fitzhugh, E.C.
Critical revision of the manuscript for important intellectual content: Steeves, J. A., Joshu, C.E., Platz, E. A. Peskoe, S., Fairchild, V., Fitzhugh, E.C., Bradwin, G., McGlynn, K. A.
Statistical analysis: Steeves, J. A., Peskoe, S., Fairchild, V., Joshu, C. E., Fitzhugh, E.C.
Obtained funding: McGlynn, K.A.
Administrative, technical, or material support: Bradwin, G.
Study supervision: Joshu, C. E., Platz, E. A.
Conflict of Interest Disclosures: There are no conflicts of interest to disclose
Previous Presentations: None
References
- Abbott RD, Launer LJ, Rodriguez BL, Ross GW, Wilson PW, Masaki KH, Strozyk D, Curb JD, Yano K, Popper JS, Petrovitch H. Serum estradiol and risk of stroke in elderly men. Neurology. 2007;68:563–68. doi: 10.1212/01.wnl.0000254473.88647.ca. [DOI] [PubMed] [Google Scholar]
- Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O’Brien WL, Bassett DR, Jr, Schmitz KH, Emplaincourt PO, Jacobs DR, Jr, Leon AS. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- Allen NE, Appleby PN, Davey GK, Key TJ. Lifestyle and nutritional determinants of bioavailable androgens and related hormones in British men. Cancer Causes Control. 2002;13:353–63. doi: 10.1023/a:1015238102830. [DOI] [PubMed] [Google Scholar]
- American College of Sports Medicine. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 1998;30:992–1008. [PubMed] [Google Scholar]
- Ari Z, Kutlu N, Uyanik BS, Taneli F, Buyukyazi G, Tavli T. Serum testosterone, growth hormone, and insulin-like growth factor-1 levels, mental reaction time, and maximal aerobic exercise in sedentary and long-term physically trained elderly males. Int J Neurosci. 2004;114:623–37. doi: 10.1080/00207450490430499. [DOI] [PubMed] [Google Scholar]
- Ballor DL, Poehlman ET. Exercise-training enhances fat-free mass preservation during diet-induced weight loss: a meta-analytical finding. Int J Obes Relat Metab Disord. 1994;18:35–40. [PubMed] [Google Scholar]
- Bernert JT, Jr, Turner WE, Pirkle JL, Sosnoff CS, Akins JR, Waldrep MK. Development and validation of sensitive method for determination of serum cotinine in smokers and nonsmokers by liquid chromatography/atmospheric pressure ionization tandem mass spectrometry. Clin Chem. 1997;43:2281–91. [PubMed] [Google Scholar]
- Blair SN. Physical inactivity: the biggest public health problem of the 21st century. Br J Sports Med. 2009;43:1–2. [PubMed] [Google Scholar]
- Breuer B, Trungold S, Martucci C, Wallenstein S, Likourezos A, Libow LS, Zumoff B. Relationships of sex hormone levels to dependence in activities of daily living in the frail elderly. Maturitas. 2001;39:147–59. doi: 10.1016/s0378-5122(01)00208-0. [DOI] [PubMed] [Google Scholar]
- Camacho EM, Huhtaniemi IT, O’Neill TW, Finn JD, Pye SR, Lee DM, Tajar A, Bartfai G, Boonen S, Casanueva FF, Forti G, Giwercman A, Han TS, Kula K, Keevil B, Lean ME, Pendleton N, Punab M, Vanderschueren D, Wu FC. Age-associated changes in hypothalamic-pituitary-testicular function in middle-aged and older men are modified by weight change and lifestyle factors: longitudinal results from the European Male Ageing Study. Eur J Endocrinol. 2013;168:445–55. doi: 10.1530/EJE-12-0890. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey: Analytic and reporting guidelines 2012a [Google Scholar]
- Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey: Questionnaires, Datasets, and Related Documentation 2012b [Google Scholar]
- Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey: Surplus sera laboratory component 2012c [Google Scholar]
- Centers for Disease Control and Prevention. Physical activity surveillance systems 2012d [Google Scholar]
- Dai WS, Kuller LH, LaPorte RE, Gutai JP, Falvo-Gerard L, Caggiula A. The epidemiology of plasma testosterone levels in middle-aged men. Am J Epidemiol. 1981;114:804–16. doi: 10.1093/oxfordjournals.aje.a113251. [DOI] [PubMed] [Google Scholar]
- Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, Bremner WJ, McKinlay JB. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87:589–98. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- Goh VH, Tong TY, Mok HP, Said B. Interactions among age, adiposity, bodyweight, lifestyle factors and sex steroid hormones in healthy Singaporean Chinese men. Asian J Androl. 2007;9:611–21. doi: 10.1111/j.1745-7262.2007.00322.x. [DOI] [PubMed] [Google Scholar]
- Gooren LJ, Behre HM, Saad F, Frank A, Schwerdt S. Diagnosing and treating testosterone deficiency in different parts of the world: results from global market research. Aging Male. 2007;10:173–81. doi: 10.1080/13685530701600885. [DOI] [PubMed] [Google Scholar]
- Gruenewald DA, Matsumoto AM. Testosterone supplementation therapy for older men: potential benefits and risks. J Am Geriatr Soc. 2003;51:101–15. doi: 10.1034/j.1601-5215.2002.51018.x. [DOI] [PubMed] [Google Scholar]
- Hall HL, Flynn MG, Carroll KK, Brolinson PG, Shapiro S, Bushman BA. Effects of intensified training and detraining on testicular function. Clin J Sport Med. 1999;9:203–8. doi: 10.1097/00042752-199910000-00004. [DOI] [PubMed] [Google Scholar]
- Handa K, Ishii H, Kono S, Shinchi K, Imanishi K, Mihara H, Tanaka K. Behavioral correlates of plasma sex hormones and their relationships with plasma lipids and lipoproteins in Japanese men. Atherosclerosis. 1997;130:37–44. doi: 10.1016/s0021-9150(96)06041-8. [DOI] [PubMed] [Google Scholar]
- Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR, Aging Baltimore Longitudinal Study of Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86:724–31. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- Hawkins VN, Foster-Schubert K, Chubak J, Sorensen B, Ulrich CM, Stancyzk FZ, Plymate S, Stanford J, White E, Potter JD, McTiernan A. Effect of exercise on serum sex hormones in men: a 12-month randomized clinical trial. Med Sci Sports Exerc. 2008;40:223–33. doi: 10.1249/mss.0b013e31815bbba9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heufelder AE, Saad F, Bunck MC, Gooren L. Fifty-two-week treatment with diet and exercise plus transdermal testosterone reverses the metabolic syndrome and improves glycemic control in men with newly diagnosed type 2 diabetes and subnormal plasma testosterone. J Androl. 2009;30:726–33. doi: 10.2164/jandrol.108.007005. [DOI] [PubMed] [Google Scholar]
- Khaw KT, Barrett-Connor E. Endogenous sex hormones, high density lipoprotein cholesterol, and other lipoprotein fractions in men. Arterioscler Thromb. 1991;11:489–94. doi: 10.1161/01.atv.11.3.489. [DOI] [PubMed] [Google Scholar]
- Khaw KT, Dowsett M, Folkerd E, Bingham S, Wareham N, Luben R, Welch A, Day N. Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: European prospective investigation into cancer in Norfolk (EPIC-Norfolk) Prospective Population Study. Circulation. 2007;116:2694–701. doi: 10.1161/CIRCULATIONAHA.107.719005. [DOI] [PubMed] [Google Scholar]
- Kuchuk NO, van Schoor NM, Pluijm SM, Smit JH, de Ronde W, Lips P. The association of sex hormone levels with quantitative ultrasound, bone mineral density, bone turnover and osteoporotic fractures in older men and women. Clin Endocrinol (Oxf) 2007;67:295–303. doi: 10.1111/j.1365-2265.2007.02882.x. [DOI] [PubMed] [Google Scholar]
- Lindholm J, Winkel P, Brodthagen U, Gyntelberg F. Coronary risk factors and plasma sex hormones. Am J Med. 1982;73:648–51. doi: 10.1016/0002-9343(82)90405-3. [DOI] [PubMed] [Google Scholar]
- Liu CC, Wu WJ, Lee YC, Wang CJ, Ke HL, Li WM, Hsiao HL, Yeh HC, Li CC, Chou YH, Huang CH, Huang SP. The prevalence of and risk factors for androgen deficiency in aging Taiwanese men. J Sex Med. 2009;6:936–46. doi: 10.1111/j.1743-6109.2008.01171.x. [DOI] [PubMed] [Google Scholar]
- Mantzoros CS, Georgiadis EI. Body mass and physical activity are important predictors of serum androgen concentrations in young healthy men. Epidemiology. 1995;6:432–35. doi: 10.1097/00001648-199507000-00020. [DOI] [PubMed] [Google Scholar]
- McHenry MC. Testosterone deficiency in older men: a problem worth treating. Consult Pharm. 2012;27:152–63. doi: 10.4140/TCP.n.2012.152. [DOI] [PubMed] [Google Scholar]
- Müller-Riemenschneider F, Meinhard C, Damm K, Vauth C. Effectiveness of nonpharmacological secondary prevention of coronary heart disease. Eur J Cardiovasc Prev Rehabil. 2010;17:688–700. doi: 10.1097/HJR.0b013e32833a1c95. [DOI] [PubMed] [Google Scholar]
- Muller M, den Tonkelaar I, Thijssen JH, Grobbee DE, van der Schouw YT. Endogenous sex hormones in men aged 40–80 years. Eur J Endocrinol. 2003;149:583–89. doi: 10.1530/eje.0.1490583. [DOI] [PubMed] [Google Scholar]
- Nigro N, Christ-Crain M. Testosterone treatment in the aging male: myth or reality? Swiss Med Wkly. 2012;142:w13539. doi: 10.4414/smw.2012.13539. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- Ohl DA, Quallich SA. Clinical hypogonadism and androgen replacement therapy: an overview. Urol Nurs. 2006;26:253–69. [PubMed] [Google Scholar]
- Paduch DA, Brannigan RE, Fuchs EF, Kim ED, Marmar JL, Sandlow JI. The laboratory diagnosis of testosterone deficiency. Urology. 2014;83:980–8. doi: 10.1016/j.urology.2013.12.024. [DOI] [PubMed] [Google Scholar]
- Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physio. 2005;98:1154–62. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- Platz EA, Giovannucci E. The epidemiology of sex steroid hormones and their signaling and metabolic pathways in the etiology of prostate cancer. J Steroid Biochem Mol Biol. 2004;92:237–53. doi: 10.1016/j.jsbmb.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Ponholzer A, Plas E, Schatzl G, Struhal G, Brössner C, Mock K, Rauchenwald M, Madersbacher S. Relationship between testosterone serum levels and lifestyle in aging men. Aging Male. 2005;8:190–93. doi: 10.1080/13685530500298154. [DOI] [PubMed] [Google Scholar]
- Rohrmann S, Platz EA, Selvin E, Shiels MS, Joshu CE, Menke A, Feinleib M, Basaria S, Rifai N, Dobs AS, Kanarek N, Nelson WG. The prevalence of low sex steroid hormone concentrations in men in the Third National Health and Nutrition Examination Survey (NHANES III) Clin Endocrinol (Oxf) 2011;75:232–39. doi: 10.1111/j.1365-2265.2011.04043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvin E, Feinleib M, Zhang L, Rohrmann S, Rifai N, Nelson WG, Dobs A, Basaria S, Golden SH, Platz EA. Androgens and diabetes in men: results from the Third National Health and Nutrition Examination Survey (NHANES III) Diabetes Care. 2007;30:234–8. doi: 10.2337/dc06-1579. [DOI] [PubMed] [Google Scholar]
- Shiels MS, Rohrmann S, Menke A, Selvin E, Crespo CJ, Rifai N, Dobs A, Feinleib M, Guallar E, Platz EA. Association of cigarette smoking, alcohol consumption, and physical activity with sex steroid hormone levels in US men. Cancer Causes Control. 2009;20:877–86. doi: 10.1007/s10552-009-9318-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas-Shankar U, Wu FC. Frailty and muscle function: role for testosterone? Front Horm Res. 2009;37:133–49. doi: 10.1159/000176050. [DOI] [PubMed] [Google Scholar]
- Stanworth RD, Jones TH. Testosterone in obesity, metabolic syndrome and type 2 diabetes. Front Horm Res. 2009;37:74–90. doi: 10.1159/000176046. [DOI] [PubMed] [Google Scholar]
- Surampudi PN, Wang C, Swerdloff R. Hypogonadism in the aging male diagnosis, potential benefits, and risks of testosterone replacement therapy. Int J Endocrinol. 2012;2012:625434. doi: 10.1155/2012/625434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svartberg J, Midtby M, Bonaa KH, Sundsfjord J, Joakimsen RM, Jorde R. The associations of age, lifestyle factors and chronic disease with testosterone in men: the Tromso Study. Eur J Endocrinol. 2003;149:145–52. doi: 10.1530/eje.0.1490145. [DOI] [PubMed] [Google Scholar]
- Tajar A, Forti G, O’Neill TW, Lee DM, Silman AJ, Finn JD, Bartfai G, Boonen S, Casanueva FF, Giwercman A, Han TS, Kula K, Labrie F, Lean ME, Pendleton N, Punab M, Vanderschueren D, Huhtaniemi IT, Wu FC. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab. 2010;95:1810–8. doi: 10.1210/jc.2009-1796. [DOI] [PubMed] [Google Scholar]
- Tajar A, Huhtaniemi IT, O’Neill TW, Finn JD, Pye SR, Lee DM, Bartfai G, Boonen S, Casanueva FF, Forti G, Giwercman A, Han TS, Kula K, Labrie F, Lean ME, Pendleton N, Punab M, Vanderschueren D, Wu FC. Characteristics of androgen deficiency in late-onset hypogonadism: results from the European Male Aging Study (EMAS) J Clin Endocrinol Metab. 2012;97:1508–16. doi: 10.1210/jc.2011-2513. [DOI] [PubMed] [Google Scholar]
- Tivesten A, Hulthe J, Wallenfeldt K, Wikstrand J, Ohlsson C. Circulating estradiol is an independent predictor of progression of carotid artery intima-media thickness in middle-aged men. J Clin Endocrinol Metab. 2006;91:4433–37. doi: 10.1210/jc.2006-0932. [DOI] [PubMed] [Google Scholar]
- U. S. Department of Health and Human Services. Physical Activity Guidelines for Americans. 2008:1–4. [Google Scholar]
- U.S. Department of Health and Human Services. Dietary Guidelines for Americans 2010 [Google Scholar]
- Wang C, Jackson G, Jones TH, Matsumoto AM, Nehra A, Perelman MA, Swerdloff RS, Traish A, Zitzmann M, Cunningham G. Low testosterone associated with obesity and the metabolic syndrome contributes to sexual dysfunction and cardiovascular disease risk in men with type 2 diabetes. Diabetes Care. 2011;34:1669–75. doi: 10.2337/dc10-2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Nieschlag E, Swerdloff R, Behre HM, Hellstrom WJ, Gooren LJ, Kaufman JM, Legros JJ, Lunenfeld B, Morales A, Morley JE, Schulman C, Thompson IM, Weidner W, Wu FC, Andrology International Society of, Male International Society for the Study of Aging, Urology European Association of, Andrology European Academy of, and Andrology American Society of Investigation, treatment, and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA, and ASA recommendations. J Androl. 2009;30:1–9. doi: 10.2164/jandrol.108.006486. [DOI] [PubMed] [Google Scholar]
- White LJ, Dressendorfer RH, Ferguson MA, Wade CE. Maintenance of testosterone status in fitness joggers after increased training mileage. Eur J Appl Physiol. 2002;86:498–502. doi: 10.1007/s00421-001-0575-z. [DOI] [PubMed] [Google Scholar]
- Yeap BB. Are declining testosterone levels a major risk factor for ill-health in aging men? Int J Impot Res. 2009;21:24–36. doi: 10.1038/ijir.2008.60. [DOI] [PubMed] [Google Scholar]
- Yeap BB, Almeida OP, Hyde Z, Norman PE, Chubb SA, Jamrozik K, Hankey GJ, Flicker L. Healthier lifestyle predicts higher circulating testosterone in older men: the Health In Men Study. Clin Endocrinol (Oxf) 2009;70:455–63. doi: 10.1111/j.1365-2265.2008.03372.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 Legend: Multivariable-adjusted association between physical activity and testosterone using restricted quadratic splines with knots at the 10th, 50th, and 90th percentiles. The top and bottom 1% of the distribution of physical activity and testosterone were excluded from the restricted quadratic splines model to avoid the influence of extreme values. Models were adjusted for age, race/ethnicity, smoking status, cotinine as a measure of exposure to environmental tobacco smoke, alcohol consumption, BMI, and waist circumference. Gray shading: Represents confidence intervals. Background: Distribution of physical activity in study population. A: All men. B: Men, 20–49 years. C: Men, ≥ 50 years. D: Non-obese men, BMI < 30 kg/m2. E: Obese men, BMI ≥ 30 kg/m2.


