Figure 18.
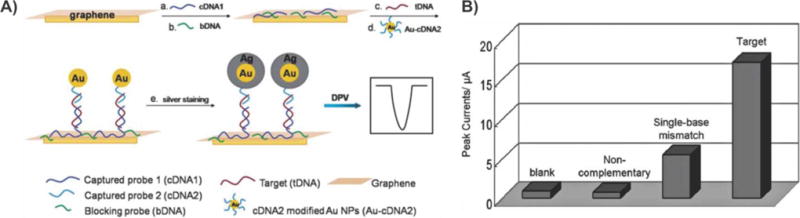
Electrochemical DNA sensor. (A) Schematic diagram. In a typical experiment, captured probe 1 (cDNA1) was adsorbed on the surface of a graphene-modified glassy carbon electrode directly (a), followed by a further adsorption of blocking probe (bDNA) (b). Different target sequences (c) and AuNPs-modified oligonucleotide probes (d) were then cohybridized to the target-active substrates in buffer solution. After silver staining (e) on AuNPs tags as a signal amplification method, a subsequent differential pulse voltammetry (DPV) technique was applied as a detection means for deposited silver. The magnitude of the anodic peak current, which corresponds to the oxidation of silver particles, reflected the amount of complementary target oligonucleotides bound to the GCE-GR/cDNA1 surface. (B) DPV responses of the electrochemical DNA sensor in the blank, noncomplementary sequence, single-base mismatch sequence, and complementary sequence. Electrolyte: 0.1 M KNO3. Pulse amplitude: 50 mV. Pulse period: 0.2 s. Silver staining time: 10 min. Reprinted with permission from ref 145. Copyright 2011 Royal Society of Chemistry.
