Table 1.
Optical properties of o-nitrophenylenthanols with various substituted groups and their corresponding oxindoles.[a]
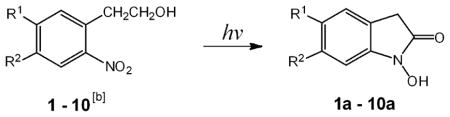
| |||||||
|---|---|---|---|---|---|---|---|
| Compds | R1 | R2 | λmax[c] [nm] | λmax[d] [nm] | λem[e] [nm] | φ[f] | F/F0[g] |
| 1–1a | COOCH3 | H | 254 | 334 | 520 | 0.36 | 800 |
| 2–2a | H | COOCH3 | 230 | 290 | 401 | 0.15 | 200 |
| 3–3a | COOCH3 | OCH3 | 334 | 321 | 502 | 0.17 | 230 |
| 4–4a | CN | H | 254 | 290 | 514 | 0.047 | 32 |
| 5–5a | CHO | H | 258 | 317 | 585 | 0.13 | 161 |
| 6–6a | COOH | H | 262 | 282 | 485 | 0.027 | 21 |
| 7–7a | H | H | 260 | 281 | 450 | 0.001 | 3 |
| 8–8a | CH2OH | H | 273 | 321 | 420 | 0.001 | 5 |
| 9–9a | OCH3 | H | 312 | 325 | 458 | 0.001 | 5 |
| 10 | H | OCH3 | 345 | 275 | 457 | 0.002 | 6 |
All the spectra were recorded in MeOH/PBS (3:1, v/v) solution with a concentration of 10 μM.
Fluorescence emission was not detected.
Absorption maxima in the 225–450 nm region for compound 1–10.
Absorption maxima in the 250–450 nm region for compound 1a–10 a.
Fluorescence emission in the 400–650 nm region.
Fluorescence quantum yields were measured using fluorescein (1a, 3a, 4a, and 5a) and DAPI (2a and 6a–10a) as standards.
Obtained by comparing the integrated area of emission.
