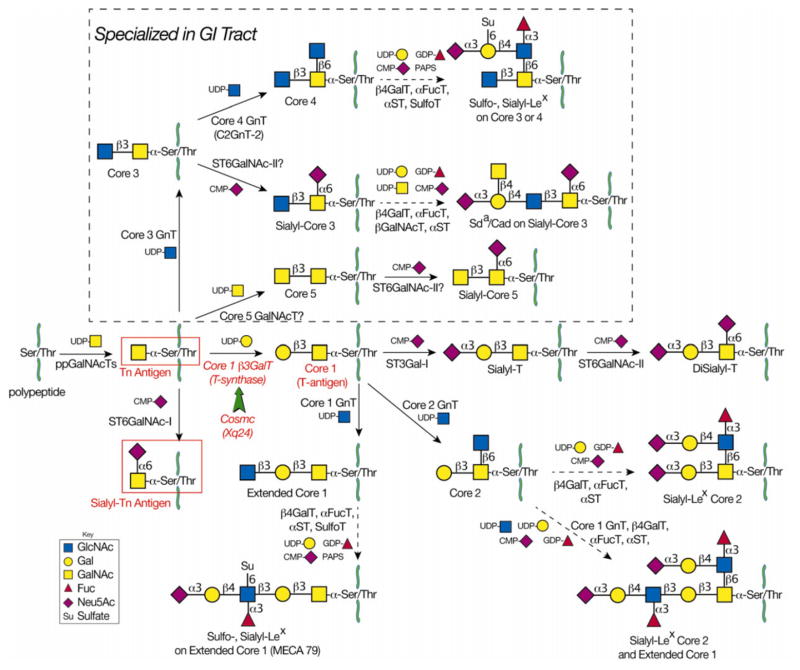
Figure 1.
The mucin-type O-glycosylation pathways. Mucin-type O-glycosylation begins in the Golgi when polypeptide GalNAc-transferases transfer GalNAc from UDP-Gal to Ser/Thr on a polypeptide chain to form Tn antigen (GalNAc-α-Ser/Thr). In nondiseased tissue, Tn antigen is further modified to form complex O-glycans. The T-synthase (Core 1 β3GalT) transfers Gal from UDP-Gal to GalNAc to form core 1 (T-antigen) in all cell types. Cosmc is the unique molecular chaperone for the T-synthase. Loss of Cosmc or T-synthase activity results in pathological expression of Tn antigen and sialyl-Tn (STn) antigen. The latter results from the actions of ST6GalNAc-I, which transfers Neu5Ac from CMP-Neu5Ac to the Tn antigen to form STn. Due to poor efficiency of ST6GalNAc-I, it is not likely that high expression of ST6GalNAc-I could outcompete functional T-synthase to result in pathologic STn expression. In GI epithelia, core 3 GnT transfers GlcNAc from UDP-GlcNAc to Tn antigen to form core 3. It is not known whether the T-synthase and core 3 GnT compete for the same substrates in GI epithelial cells. The T antigen is further modified to form extended core 1 structures. Similarly, core 3 is further modified to form extended core 3 structures, including sulfo-Lex, sialyl-Lex, or Sda on core 3 or core 4 O-glycans.