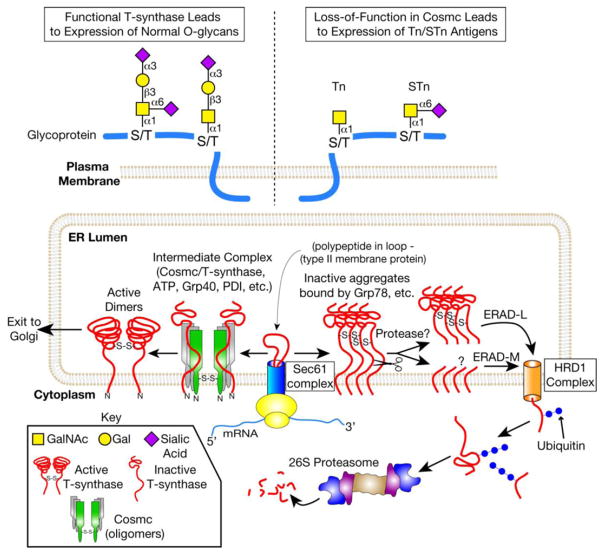
Figure 2.
The major molecular mechanism for the expression of Tn and STn antigens in cells lacking a functional Cosmc. Cosmc is the unique molecular chaperone for the T-synthase. Nascent T-synthase is translocated to the ER possibly via the Sec61 complex. Cosmc interacts cotranslationally with nascent, nonnative T-synthase to form active, dimeric T-synthase. The T-synthase is subsequently transported to the Golgi. In the Golgi, the T-synthase transfers Gal from UDP-Gal to Tn antigen to form the T antigen on polypeptide chains. Defective Cosmc due to genetic or epigenetic alterations in Cosmc (e.g. ORF mutations, promoter methylation, or loss of heterozygosity) results in aggregation and proteosomal degradation of the T-synthase. Mis-folded T-synthase interacts with Grp78, is cleaved in its lumenal domain by an unknown protease, and is retrotranslocated to the cytosol. In the cytosol, soluble T-synthase is polyubiquitinated and degraded by the 26S proteasome. Loss of T-synthase activity results in expression of Tn and sialylTn antigens, which are not present in normal, non-transformed tissue.