Figure 18.
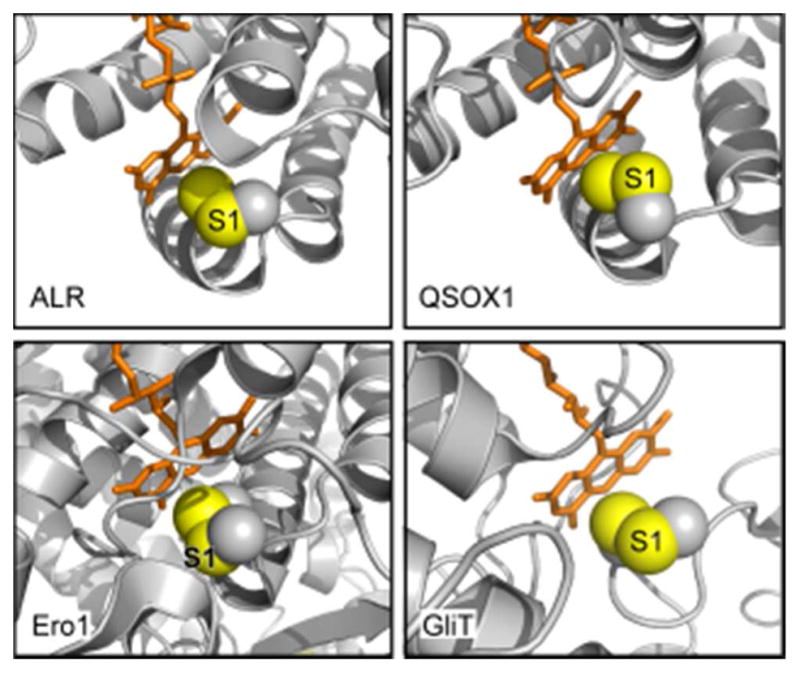
Active-site regions of flavin-dependent sulfhydryl oxidases. Flavin-proximal disulfides are shown as spheres with sulfur atoms in yellow and Cβ atoms in gray. The sulfur in each enzyme targeted for nucleophilic attack by an incoming thiolate during catalysis (the interchange sulfur) is labeled S1. The relative solvent exposure of the S1 sulfurs and accessibility of the disulfide to in-line attack is evident in ALR, QSOX1, and GliT. Only in Ero1 is the FAD-proximal disulfide buried and the S1 cysteine apparently inaccessible. PDB files are ALR, 1OQC; QSOX1, 3LLI; Ero1, 1RP4; GliT, 4NTC.
