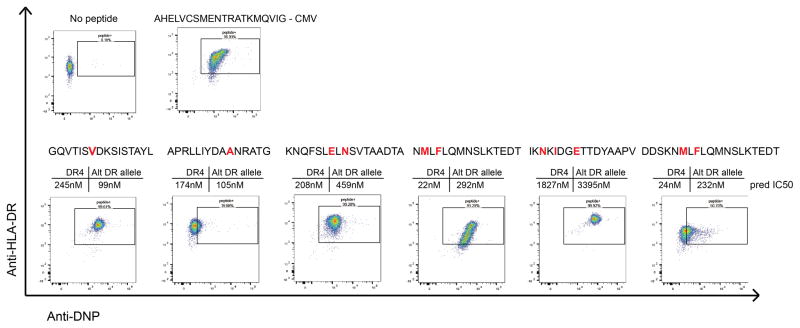
Extended Data Figure 7. Experimental determination affinity of HLA-DRB1*04:01 with associated immunoglobulin neoantigens.
Six neoantigen peptides identified from 3 patients were synthesized with an N-terminal DNP modification and tested for binding to recombinant HLA-DR4 molecules. Recombinant, biotinylated HLA-DR4 molecules were produced with a thrombin-cleavable CLIP peptide. Neoantigen peptides were exchanged unto the DR4 molecules. HLA-DR4 molecules were then bound to streptavidin coated microsphere beads and co-stained anti-HLA-DR antibody and anti-DNP antibody. Beads were then washed and analyzed by flow cytometry for dual staining against HLA-DR and DNP-labelled peptide. A known CMV-derived peptide ligand of HLA-DR4 was used as a positive control. Shown above each plot is the predicted affinity of each peptide for both HLA-DR4 and the associated patient’s alternative HLA-DR allele as predicted by netMHCII. Red letters indicate amino acids that differ from the germline variable gene sequence due to somatic hypermutation events.