Abstract
Background
In a prior agent-based modeling study, offering a choice of influenza vaccine type was shown to be cost-effective when the simulated population represented the large, Washington DC metropolitan area. This study calculated the public health impact and cost-effectiveness of the same four strategies: No Choice, Pediatric Choice, Adult Choice, or Choice for Both Age Groups in five United States (U.S.) counties selected to represent extremes in population age distribution.
Methods
The choice offered was either inactivated influenza vaccine delivered intramuscularly with a needle (IIV-IM) or an age-appropriate needle-sparing vaccine, specifically, the nasal spray (LAIV) or intradermal (IIV-ID) delivery system. Using agent-based modeling, individuals were simulated as they interacted with others, and influenza was tracked as it spread through each population. Influenza vaccination coverage derived from Centers for Disease Control and Prevention (CDC) data, was increased by 6.5% (range 3.25%–11.25%) to reflect the effects of vaccine choice.
Results
Assuming moderate influenza infectivity, the number of averted cases was highest for the Choice for Both Age Groups in all five counties despite differing demographic profiles. In a cost-effectiveness analysis, Choice for Both Age Groups was the dominant strategy. Sensitivity analyses varying influenza infectivity, costs, and degrees of vaccine coverage increase due to choice, supported the base case findings.
Conclusion
Offering a choice to receive a needle-sparing influenza vaccine has the potential to significantly reduce influenza disease burden and to be cost saving. Consistent findings across diverse populations confirmed these findings.
Keywords: Influenza, Influenza vaccine, Cost-effectiveness, Vaccine choice, Agent-based modeling
1. Introduction
Needle aversion is one among many reasons cited for not receiving influenza and other vaccines. Needle-sparing vaccine administration such as nasal spray, intradermal, or micro-needle patch delivery methods increase vaccine acceptability [1–7]. Over time, the use of these vaccine types may create a larger demand for influenza vaccine, increase overall vaccine uptake and reduce influenza morbidity, as well as mortality. Offering a choice may increase vaccination rates, but the costs of newer influenza vaccines, the extra effort required to order and stock several types of influenza vaccine, the inability to predict the demand for any given type, and the time required to explain the benefits of each option to patients may discourage providers from offering a choice. Without evidence that offering a choice of influenza vaccines is cost-effective, the benefits of new influenza vaccine options may not be realized.
In a previous study, the cost-effectiveness of offering age-appropriate choice of influenza vaccines to adults and children was examined using decision analysis (DA) modeling combined with agent-based modeling (ABM). When vaccine coverage increased by 3.25% or more as a result of offering a choice of vaccines, influenza cases decreased by >100,000 in a simulated Washington DC metropolitan area population, and the strategy was cost-effective [8]. That synthetic population had a large proportion of young adults. It was unknown whether a similar analysis using other U.S. metropolitan areas would yield similar results.
Using ABM and DA, this study was undertaken to examine the cost-effectiveness of offering a choice of needle-sparing influenza vaccines using population data from five U.S. counties, i.e., Allegheny County, Pennsylvania, Wayne County, Michigan, Santa Clara County, California, Sacramento County, California and Salt Lake County, Utah. Each county surrounds an urban center and was selected because combined, they varied by age distribution, geography and socioeconomic structure. ABM simulates the spread of influenza through spatially and temporally heterogeneous interactions among individuals in synthetic populations representing each county while varying vaccination coverage, accounting for vaccine choice and vaccine-induced herd immunity. This report assesses the public health outcomes and cost-effectiveness of three choice strategies across five U.S. counties, each selected to represent extremes in population age distribution.
2. Methods
The study was conducted in two steps. First, a previously published agent-based model (ABM) [9–12] was used to predict epidemiological outcomes in each of the five counties during a single influenza season under various vaccine choice options. Second, using those results, cost-effectiveness (CE) was analyzed from a societal perspective. This study did not require IRB approval because it did not involve human subjects and only secondary, anonymous data were used for model parameterization. The methods have been previously published [8], but are briefly described herein.
2.1. Selected geographical areas
In an effort to select a group of counties with varying population characteristics, non-metric multidimensional scaling was performed on the matrix of dissimilarities in age distributions for all U.S. counties published by the U.S. Census Bureau [13,14]. Selections of counties were based on informal visual identification of the most widely separated coordinates in the 2-dimensional matrix, confirmed by population age histograms. Demographic characteristics of each of the counties are shown in Table 1. The areas, though similar in size, varied across a range of population densities; the proportion of children, working age adults and older adults and average age; the number and density of workplaces.
Table 1.
Characteristics of five U.S. counties.
| Demographic characteristic | County, State
|
||||
|---|---|---|---|---|---|
| Allegheny, PA | Wayne, MI | Santa Clara, CA | Sacramento, CA | Salt Lake, UT | |
| Population, n | 1,164,879 | 1,778,979 | 1,604,122 | 1,315,644 | 941,139 |
| Household size, mean | 2.22 | 2.51 | 2.74 | 2.61 | 2.85 |
| Schools, n | 532 | 813 | 615 | 532 | 409 |
| School size, mean number of students | 373 | 479 | 508 | 520 | 520 |
| Work places, n | 48,704 | 68,281 | 68,975 | 60,318 | 48,000 |
| Workplace size, mean number of workers | 11.8 | 10.5 | 11.4 | 10.2 | 10.0 |
| Age, mean years | 40.3 | 36.3 | 35.8 | 35.2 | 32.8 |
| Proportion aged 0–18 years, % | 21.7 | 28.1 | 25.7 | 27.6 | 30.8 |
| Proportion aged 19–65 years, % | 62.0 | 60.2 | 64.0 | 61.9 | 61.1 |
| Proportion aged 65+ years, % | 16.2 | 11.6 | 10.1 | 10.4 | 8.0 |
| Influenza attack rate from Figs. 1a–1d | 11.1 | 15.0 | 15.0 | 15.3 | 17.8 |
2.2. Agent-based model
Using a version of the FRED (Framework for Reconstructing Epidemic Dynamics) ABM [15], simulations were conducted for the five geographical areas, Allegheny County, Pennsylvania, Wayne County, Michigan, Santa Clara County, California, Sacramento County, California and Salt Lake County, Utah, using synthetic populations based on the 2010 U.S. Census [16]. Virtual people (i.e., “agents”) were assigned to households, schools and work places that represented the geospatial population density and demographics for each census tract, and came into contact with each other in those locations and in the community [17]. The rate of contact between agents is a function of both the type of place where contact occurs (e.g., household or school), as well as the age of each agent in the contact pair.
2.3. Influenza transmission
On a given day, each agent may be in one of four influenza disease states: susceptible, exposed, infectious, or recovered. The influenza epidemic was “seeded” by randomly selecting 100 agents and assigning them to the infectious state. When exposed to influenza, a susceptible agent moves to the exposed state for a latent period drawn from a truncated Weibull distribution with a mean of 1.9 days, after which the agent moves to the infectious state for an infectious period drawn from a truncated Weibull distribution with a mean of 4.1 days. One third (33%) of the agents who enter the infectious state are asymptomatic and are assumed to be 50% less infectious than a symptomatic agent [18,19]. Half (50%) of all symptomatic agents are assumed to stay home from school or work. These parameters and assumptions are consistent with previously published ABM studies [17,20–25].
The transmission of influenza was calibrated according to the procedure described by Grefenstette et al. [15], based on age-specific attack rates derived from Molinari et al. [26], which were translated to the basic reproduction number (R0) of 1.3, labeled “Moderate Infectivity.” Parameters for the ABM transmission model are summarized in Table 2. In sensitivity analyses, transmissibility was varied from R0 = 1.0 (Mild Infectivity) to R0 = 1.6 (High Infectivity).
Table 2.
Key parameters in the agent-based model.
| Parameter | % | Vaccine received (%)
|
Range
|
Reference | ||||
|---|---|---|---|---|---|---|---|---|
| LAIV | IIV-IM | IIV-ID | No. | Minimum | Maximum | |||
| Coverage by age, years | ||||||||
| 6–23 months | 70.4 | 0.0 | 100.0 | 0.0 | 69.6 | 71.2 | Adapted from [38,39] | |
| 2–4 | 68.1 | 52.0 | 48.0 | 0.0 | 67.3 | 68.9 | ||
| 5–8 | 61.0 | 52.0 | 48.0 | 0.0 | 60.2 | 61.8 | ||
| 9–17 | 52.9 | 52.0 | 48.0 | 0.0 | 52.1 | 53.7 | ||
| 18–49 | 32.3 | 2.0 | 97.0 | 1.0 | 31.9 | 32.7 | ||
| 50–64 | 45.3 | 0.0 | 99.0 | 1.0 | 44.9 | 45.7 | ||
| 65–106 | 65.0 | 0.0 | 100.0 | 0.0 | 64.6 | 65.4 | ||
| LAIV contraindications by age, years | ||||||||
| 2–4 | 8.8 | [40] | ||||||
| 5–17 | 1.0 | |||||||
| Absolute vaccine coverage increases with choice | 6.5 | 3.25 | 11.25 | [1] | ||||
| Vaccine cost, US$ | ||||||||
| IIV-IM | 10.69 | 0.00 | 21.38 | [41] | ||||
| LAIV | 23.70 | 0.00 | 47.40 | |||||
| IIV-ID | 15.69 | 0.00 | 31.38 | |||||
| Vaccine administration cost, US$ | 25.08 | [28] | ||||||
| Increased cost of choice | 5.0 | 2.5 | 7.5 | [42] | ||||
| Latent period, daysa | 1.9 | 1 | 2 | [17,20–25,43] | ||||
| Infectious period, daysa | 4.1 | 3 | 6 | |||||
| Asymptomatic rateb | 33.0 | |||||||
| Asymptomatic reduction in infectivityc | 50.0 | |||||||
| Symptomatic absenteeismb | 50.0 | |||||||
| Vaccine effectivenessb | 59.0 | [44] | ||||||
IIV = inactivated influenza vaccine; LAIV = live attenuated influenza vaccine; IM = intramuscular; ID = intradermal.
Drawn from a truncated Weibull distribution with the listed nominal (mean), minimum and maximum values.
Probability point estimate that does not vary across simulations; within each simulation, it is the action potential threshold above which an outcome (i.e., exhibit symptoms, stay home from work, or vaccine protects against disease) occurs and below which it does not.
A constant that does not vary across simulations.
2.4. Vaccine effectiveness and choice
Each vaccine had an age-specific vaccine effectiveness, defined as the probability of that vaccine producing full and permanent immunity against influenza 14 days after its receipt. Probability of remaining susceptible was equal to 1 minus the vaccine effectiveness. Because this study modeled only a single season, waning immunity was not considered.
The baseline vaccination strategy, wherein adults and children accepted the only offered vaccine, was designated No Choice; the likelihood of vaccination was the mean observed monthly vaccination rate over five influenza seasons from 2009–10 to 2013–14 [27]. Three additional strategies were considered, where choice between two age-appropriate alternative vaccines led to increased vaccination among recipients of the needle-sparing vaccine. In the Pediatric Choice strategy, children aged 2–18 years “chose” between IIV-IM and LAIV based on a weighted probability draw that ensures the appropriate proportion of children receiving vaccines matches the overall increased coverage and the increased usage of needle-sparing vaccine. Whereas, in the Adult Choice strategy, this procedure was performed for adults aged 19–65 years who “chose” between IIV-IM or IIV-ID. Both LAIV and IIV-ID are needle-sparing vaccine delivery options. The fourth strategy was Choice in Both Age Groups, where options were offered to both adults and children.
Table 2 provides coverage distributions for all strategies. For each choice strategy, the vaccine coverage was assumed to be 6.5% greater [1] than the No Choice baseline strategy. The same value was used across counties because our purpose was to evaluate the model in different settings, each with its own age pyramid and spatial structure. In sensitivity analyses, changes in vaccine coverage due to choice were varied between 3.25% and 11.25%.
Using the FRED ABM, 756 simulations were conducted in total. For each vaccination strategy, 189 individual simulations (accounting for inherent stochasticity as well as sensitivity analyses) were averaged, resulting in age-specific numbers of influenza cases. These estimates were then used for the CE analyses. All simulations were run on the Olympus High Performance Computing Cluster at the Pittsburgh Supercomputing Center.
2.5. Data sources and cost-effectiveness
The age-specific population likelihoods of influenza disease were derived from the ABM as described above. The epidemiological outcomes simulated using the FRED ABM were then used as inputs for the DA model for the cost-effectiveness analysis. Specifically, a DA model was used to calculate the incremental cost-effectiveness ratio (ICER), i.e., the difference in cost between strategies divided by the difference in effectiveness between strategies, measured as quality-adjusted life years (QALYs) lost for each strategy, in each county, from a societal perspective.
Costs and health outcomes for the CE analysis were derived from several sources and included the costs associated with vaccination and influenza disease outcomes, including outpatient and all hospitalized and fatal cases. Total influenza vaccination costs included base costs of each vaccine multiplied by the likelihood of vaccination, adjusted by the proportion of the population vaccinated with each vaccine under the three experimental strategies. Unit costs of vaccines are shown in Table 2. Vaccination costs also included fixed administration costs common to all vaccines of $25.08, based on Medicare reimbursement data [28], irrespective of the recipient’s age. Where choice occurred, based on expert judgment for extra overhead costs, an additional 5% administrative and 5% material cost, (varied between 2.5 and 7.5% in sensitivity analyses) was added to each respective vaccine cost component in the ABM. These values were based on cost of vaccine administration data in pediatric and adult medical practices [29,30]. To be conservative, the total vaccination cost in choice strategies, as calculated by the ABM was also varied in sensitivity analyses from 0 to 200% of the ABM output in the DA model. Average influenza costs per case (Appendix Table 1) were calculated from published data [26] after inflating to 2014 U.S. dollars using the U.S. Consumer Price Index [8].
For the calculation of ICER, lost QALYs for non-hospitalized cases were estimated based on published data on the probabilities of being a high risk case and/or requiring an office visit, and their respective days of lost productivity [26]. QALY losses due to influenza hospitalization were similarly based on days of lost productivity due to that event [26]. Lifetime QALYs lost due to fatal influenza were estimated based on age-group-specific life expectancies from U.S. life tables and discounted at 3% per year. In sensitivity analyses, all influenza-illness-related disutilities were varied from 50 to 150% from their base case values.
3. Results
As shown in Figs. 1a–1d, the influenza epidemic curves for the five counties differed considerably. Salt Lake County, with its younger population had the highest attack rates and Allegheny County, with its older population had the lowest attack rate in all vaccination strategies. The epidemic curves for the other three counties were similar. When No Choice was compared with the other three choice strategies, the epidemic peaked sooner and at a higher level for all counties. Reductions in attack rates ranged from 5.8 to 9.0 percentage points for the Choice for Both Age Groups strategy. Fig. 2 shows the number of averted cases as a fraction of the population in each age interval for each county, as a result of each choice strategy. In every county, the Choice for Both Groups averted the most cases among children followed by the Adult Choice and then the Pediatric Choice strategy.
Fig. 1.
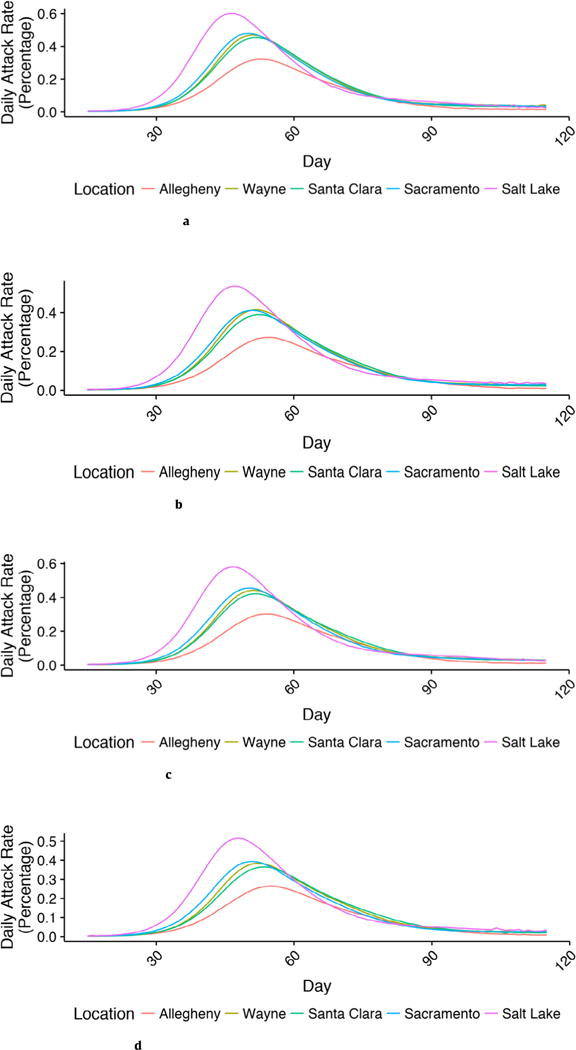
a. Attack rate curves by location: no choice.
b. Attack rate curves by location: adult choice.
c. Attack rate curves by location: pediatric choice.
d. Attack rate curves by location: choice for both age groups.
Fig. 2.
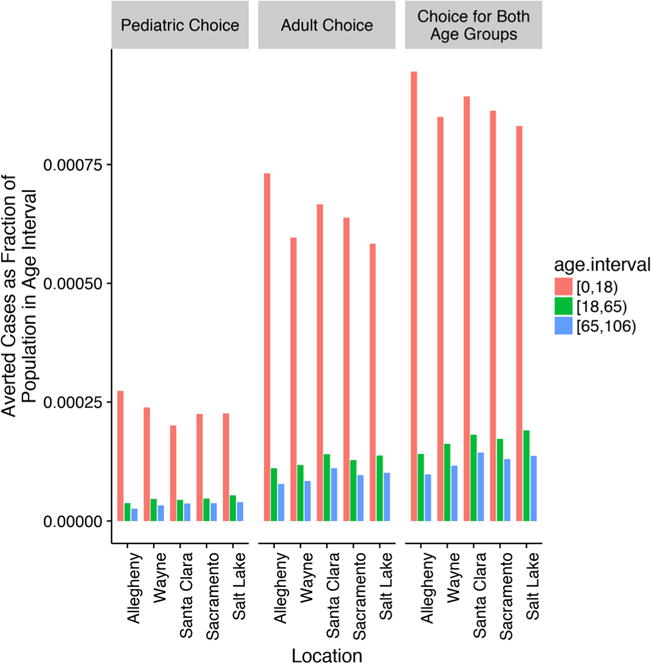
Averted cases by age interval and county. In Allegheny County, relative to the No Choice strategy, the number of averted cases was 5494 in the Pediatric Choice, 15,581 in the Adult Choice, and 19,948 in the Choice for Both Age Groups strategies. In Wayne County, relative to the No Choice strategy, the number of averted cases was 9665 in the Pediatric Choice, 24,303 in the Adult Choice, and 34,054 in the Choice for Both Age Groups strategies. In Santa Clara County, relative to the No Choice strategy, the number of averted cases was 7819 in the Pediatric Choice, 25,245 in the Adult Choice, and 33,154 in the Choice for Both Age Groups strategies. In Sacramento County, relative to the No Choice strategy, the number of averted cases was 7069 in the Pediatric Choice, 19,445 in the Adult Choice, and 26,296 in the Choice for Both Age Groups strategies. In Salt Lake County, relative to the No Choice strategy, the number of averted cases was 5597 in the Pediatric Choice, 14,316 in the Adult Choice, and 20,040 in the choice for both strategies.
3.1. Cost-effectiveness analysis
In the cost-effectiveness analysis using the burden of disease data from the ABM, the Choice for Both Age Groups strategy was less costly and more effective (i.e., dominant) compared with strategies offering choice to adults only, children only, or no choice at all. Table 3 shows the QALYs lost and incremental costs and effectiveness for the four choice strategies for the base case of 6.5% increased vaccine coverage.
Table 3.
Cost-effectiveness of each choice strategy when vaccine choice increases coverage by 6.5% in five U.S. counties.
| U.S. county | Choice strategy | Cost per person | Incremental cost | Effectiveness (QALYs) | Incremental cost-effectiveness ratio |
|---|---|---|---|---|---|
| Allegheny | Choice for both age groups | $48.43 | – | −0.00152 | – |
| Adult choice | $49.12 | $0.69 | −0.00157 | Dominated | |
| Pediatric choice | $52.98 | $4.55 | −0.00172 | Dominated | |
| No choice | $53.88 | $5.45 | −0.00178 | Dominated | |
| Wayne | Choice for both age groups | $57.47 | – | −0.00168 | – |
| Adult choice | $58.82 | $1.34 | −0.00175 | Dominated | |
| Pediatric choice | $62.36 | $4.89 | −0.00188 | Dominated | |
| No choice | $63.71 | $6.24 | −0.00195 | Dominated | |
| Santa Clara | Choice for both age groups | $62.33 | – | −0.00171 | – |
| Adult choice | $63.97 | $1.64 | −0.00179 | Dominated | |
| Pediatric choice | $67.20 | $4.88 | −0.00190 | Dominated | |
| No choice | $68.66 | $6.34 | −0.00197 | Dominated | |
| Sacramento | Choice for both age groups | $58.18 | – | −0.00173 | – |
| Adult choice | $59.32 | $1.14 | −0.00179 | Dominated | |
| Pediatric choice | $63.55 | $5.38 | −0.00194 | Dominated | |
| No choice | $64.85 | $6.67 | −0.00202 | Dominated | |
| Salt Lake | Choice for both age groups | $56.86 | – | −0.00168 | – |
| Adult choice | $58.07 | $1.21 | −0.00175 | Dominated | |
| Pediatric choice | $61.38 | $4.52 | −0.00187 | Dominated | |
| No choice | $62.74 | $5.88 | −0.00194 | Dominated |
QALY = quality adjusted life year; CE = cost-effectiveness.
To test the robustness of these results, we performed another series of sensitivity analyses within the DA model. When coverage increased 6.5% through vaccine choice, individual variations in illness costs, hospitalization or mortality risk, or QALYs lost due to illness events, did not affect the dominance of the Choice for Both Age Groups strategy over other strategies. Averaged across all models considered for each location, influenza cases would need to be reduced by more than 94% (due to a perhaps implausibly mild influenza season) to make Choice for Both Age Groups become a non-dominant strategy. The other sensitive parameter was vaccination cost for choice strategies. Vaccination costs were computed from the number of vaccinations reported by the ABM combined with the cost of administering vaccines showing that the additional costs of offering a choice of vaccines was determined to be $5–$26 per person, depending upon the age group. For all locations considered, Choice for Both Age Groups was no longer cost saving when the vaccination cost multiplier exceeded 1.75 and vaccine choice increased coverage by at least 6.5% (base case). In Santa Clara County, the Choice for Both Age Groups strategy was least cost-saving. However, even when vaccination costs were doubled, this strategy cost $39,128 per QALY gained compared to other strategies. This value is well within contemporary U.S. cost-effectiveness benchmarks of $100,000 or more per QALY gained [31]. Similar results (Table 3) occurred in all locations when the other choice-related vaccine coverage scenarios were similarly tested. The recently revised recommendations of the Panel on Cost-Effectiveness in Health and Medicine state that the reference case analysis from a societal perspective should include lost productivity due to illness [32]. These analyses did not include lost productivity. If the costs of lost productivity due to influenza were included, using the base case values in Appendix Tables 2 and 3, the incremental cost differences between the various strategies listed in Table 3 approximately tripled, making the Choice for Both Age Groups strategy even more cost saving compared to the other strategies, strengthening the case for the choice interventions.
4. Discussion
In previous research using a simulated population of the large Washington, DC metropolitan area, we have reported that offering a choice of influenza vaccine type with its presumed increase in vaccine uptake significantly decreased disease burden by reducing the number of influenza cases [8]. In this study, we examined five metropolitan areas that are smaller than metro Washington, DC and differ from each other to represent the geographic and demographic diversity of the United States. In each county, the earlier results were confirmed: offering a choice of vaccines for all vaccinees, i.e., the Choice for Both Age Groups strategy, averted the largest number of influenza cases overall. Even though having a choice of vaccines has more upfront costs, Choice for Both Age Groups dominated the other strategies. The findings were robust and cost-effective even when the baseline risk of influenza or the cost of vaccination was changed.
Recent reports of ineffectiveness of the LAIV against influenza A strains [33,34], led the Advisory Committee on Immunization Practices in June 2016 to recommend against use of the LAIV for the 2016–2017 influenza season, eliminating vaccine choice for individuals 2–17 years old. This fact does not negate the relevance of the findings of this study. Nowalk et al. found that offering a choice of vaccines (LAIV or IIV-IM) to workers 18–49 years significantly increased uptake of vaccine [1]. Although LAIV is not currently an option, IIV-ID another needle-sparing option, may be used among adults 18–64 years of age and hence, may similarly increase influenza vaccine uptake.
In the current study, offering a choice of vaccines to adults, whether in combination with children or not, resulted in the greatest reductions in influenza disease, especially among children. These results indicate the indirect benefits experienced by children due to increased vaccine coverage among adults. Moreover, the indirect effects were most pronounced in Salt Lake County, which had the highest proportion of children and the lowest mean age. This protection of children through increased vaccine uptake as a result of vaccine choice among adults is a form of cocooning, a strategy in which unvaccinated, but vulnerable individuals are protected from disease through herd immunity provided by vaccination of those with whom they come into contact. These results suggest that a similar cocooning strategy may be applicable to influenza, consistent with previous findings [35,36].
The impact of vaccine choice on disease burden should not be underestimated, particularly given influenza’s significant annual morbidity and mortality. Our data are augmented by survey data [2–7,37] and a clinical trial of the impact of choice on vaccination coverage [1]. We believe that public health leaders and medical managers should consider methods to increase vaccine choice, towards the greater purposes of higher vaccine coverage and prevention of disease, when effective vaccines are available.
4.1. Strengths and limitations
ABM is time-consuming to run on a supercomputer cluster; hence, the number of sensitivity analyses was limited. Nevertheless, in all counties of differing demographic patterns, the results were consistent and insensitive to variations in the parameters tested. The ABM employed here is influenced by other location-specific factors beyond age such as the distribution of household size. As the cost of computation continues to decrease with advances in computing hardware, it may become possible to perform this study using a larger fraction of or the entirety of the U. S. population. All simulations are subject to the parameters included; thus, we used moderate estimates of vaccine effectiveness derived from meta-analyses; we did not include the international debate on LAIV effectiveness because it has not yet been reflected in published meta-analyses. If LAIV continues to be not recommended in the U.S., as in the 2016–2017 season, a needle-sparing option might not be available to U.S. children.
5. Conclusions
Using an ABM, we found that offering a choice of influenza vaccines that included needle-sparing options for adults and children, assuming even moderate increases in coverage, reduced costs and decreased influenza cases by 5600–35,000 across the five counties tested, with populations ranging from 940,000 to 1.8 million. Of particular note, providing adults with vaccine choice reduced influenza in children. Therefore, public health officials and medical leaders should consider policies and procedures to facilitate needle-sparing influenza vaccine choice especially among adults.
Supplementary Material
Acknowledgments
Funding
Research was supported by the National Institute of General Medical Sciences of the National Institutes of Health (NIH) [grant number R01GM111121]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Richard Zimmerman has active research grants from Sanofi Pasteur, Pfizer Inc. and Merck & Co., Inc. Mary Patricia Nowalk currently receives grant funding from Pfizer, Inc. and Merck & Co., Inc. Jonathan Raviotta currently receives grant funding from Pfizer, Inc. and Merck & Co., Inc.
Abbreviations
- LAIV
live attenuated influenza vaccine
- IIV
inactivated influenza vaccine
- IM
intramuscular
- ID
intradermal
- CE
cost-effectiveness
- ABM
agent-based model
- QALY
quality-adjusted life year
Appendix A. Supplementary material
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.vaccine.2017.05.093.
Footnotes
Clinical trial number
Not applicable.
Potential conflicts of interest
The other authors have no conflicts to report.
References
- 1.Nowalk MP, Lin CJ, Toback SL, Rousculp MD, Eby C, Raymund M, Zimmerman RK. Improving influenza vaccination rates in the workplace: a randomized trial. Am J Preventive Med. 2010;38(3):237–46. doi: 10.1016/j.amepre.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 2.Flood EM, Block SL, Hall MC, Rousculp MD, Divino VM, Toback SL, Mahadevia PJ. Children’s perceptions of influenza illness and preferences for influenza vaccine. J Pediatric Health Care: Official Publication National Assoc Pediatric Nurse Assoc Practitioners. 2011;25(3):171–9. doi: 10.1016/j.pedhc.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 3.Flood EM, Ryan KJ, Rousculp MD, Beusterien KM, Block SL, Hall MC, Mahadevia PJ. A survey of children’s preferences for influenza vaccine attributes. Vaccine. 2011;29(26):4334–40. doi: 10.1016/j.vaccine.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 4.Flood EM, Ryan KJ, Rousculp MD, Beusterien KM, Divino VM, Block SL, Hall MC, Mahadevia PJ. Parent preferences for pediatric influenza vaccine attributes. Clin Pediatr (Phila) 2011;50(4):338–47. doi: 10.1177/0009922810391247. [DOI] [PubMed] [Google Scholar]
- 5.Arnou R, Frank M, Hagel T, Prebet A. Willingness to vaccinate or get vaccinated with an intradermal seasonal influenza vaccine: a survey of general practitioners and the general public in France and Germany. Adv Therapy. 2011;28(7):555–65. doi: 10.1007/s12325-011-0035-z. [DOI] [PubMed] [Google Scholar]
- 6.Reygrobellet C, Viala-Danten M, Meunier J, Weber F, Nguyen VH. Perception and acceptance of intradermal influenza vaccination: Patient reported outcomes from phase 3 clinical trials. Hum Vaccin. 2010;6(4):336–45. doi: 10.4161/hv.6.4.10753. [DOI] [PubMed] [Google Scholar]
- 7.Norman JJ, Arya JM, McClain MA, Frew PM, Meltzer MI, Prausnitz MR. Microneedle patches: usability and acceptability for self-vaccination against influenza. Vaccine. 2014;32(16):1856–62. doi: 10.1016/j.vaccine.2014.01.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DePasse J, Smith KJ, Raviotta JM, Shim E, Nowalk MP, Zimmerman RK, Brown ST. Does choice of influenza vaccine type change disease burden and cost-effectiveness in the US? An agent-based modeling study. Am J Epidemiol. 2017;185(9):822–31. doi: 10.1093/aje/kww229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee BY, Brown ST, Bailey RR, Zimmerman RK, Potter MA, McGlone SM, Cooley PC, Grefenstette JJ, Zimmer SM, Wheaton WD, et al. The benefits to all of ensuring equal and timely access to influenza vaccines in poor communities. Health Affairs. 2011;30(6):1141–50. doi: 10.1377/hlthaff.2010.0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee BY, Brown ST, Cooley P, Grefenstette JJ, Zimmerman RK, Zimmer SM, Potter MA, Rosenfeld R, Wheaton WD, Wiringa AE, et al. Vaccination deep into a pandemic wave potential mechanisms for a “third wave” and the impact of vaccination. Am J Preventive Med. 2010;39(5):E21–9. doi: 10.1016/j.amepre.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee BY, Brown ST, Cooley PC, Zimmerman RK, Wheaton WD, Zimmer SM, Grefenstette JJ, Assi TM, Furphy TJ, Wagener DK, et al. A computer simulation of employee vaccination to mitigate an influenza epidemic. Am J Preventive Med. 2010;38(3):247–57. doi: 10.1016/j.amepre.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee BY, Brown ST, Korch GW, Cooley PC, Zimmerman RK, Wheaton WD, Zimmer SM, Grefenstette JJ, Bailey RR, Assi TM, et al. A computer simulation of vaccine prioritization, allocation, and rationing during the 2009 H1N1 influenza pandemic. Vaccine. 2010;28(31):4875–9. doi: 10.1016/j.vaccine.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patterns of metropolitan and micropolitan population change: 2000 to 2010. [ https://www.census.gov/library/visualizations/2012/dec/c2010sr-01-pyramid.html]
- 14.Wilson SG. Patterns of metropolitan and micropolitan population change: 2000 to 2010: US Department of Commerce. US Census Bureau: Economics and Statistics Administration; 2012. [Google Scholar]
- 15.Grefenstette JJ, Brown ST, Rosenfeld R, DePasse J, Stone NT, Cooley PC, Wheaton WD, Fyshe A, Galloway DD, Sriram A, et al. FRED (a Framework for Reconstructing Epidemic Dynamics): an open-source software system for modeling infectious diseases and control strategies using census-based populations. BMC Public Health. 2013;13:940. doi: 10.1186/1471-2458-13-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. (RTI Press Publication No. MR-0010-0905).Synthesized population databases: A US geospatial database for agent-based models: A US geospatial database for agent-based models. doi: 10.3768/rtipress.2009.mr.0010.0905. [ http://www.rti.org/pubs/mr-0010-0905-wheaton.pdf] [DOI] [PMC free article] [PubMed]
- 17.Lee BY, Tai JH, Bailey RR, Smith KJ. The timing of influenza vaccination for older adults (65 years and older) Vaccine. 2009;27(50):7110–5. doi: 10.1016/j.vaccine.2009.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elveback LR, Fox JP, Ackerman E, Langworthy A, Boyd M, Gatewood L. An influenza simulation model for immunization studies. Am J Epidemiol. 1976;103(2):152–65. doi: 10.1093/oxfordjournals.aje.a112213. [DOI] [PubMed] [Google Scholar]
- 19.Longini IM, Jr, Halloran ME, Nizam A, Yang Y. Containing pandemic influenza with antiviral agents. Am J Epidemiol. 2004;159(7):623–33. doi: 10.1093/aje/kwh092. [DOI] [PubMed] [Google Scholar]
- 20.Ferguson NM, Cummings DA, Cauchemez S, Fraser C, Riley S, Meeyai A, Iamsirithaworn S, Burke DS. Strategies for containing an emerging influenza pandemic in Southeast Asia. Nature. 2005;437(7056):209–14. doi: 10.1038/nature04017. [DOI] [PubMed] [Google Scholar]
- 21.Ferguson NM, Cummings DA, Fraser C, Cajka JC, Cooley PC, Burke DS. Strategies for mitigating an influenza pandemic. Nature. 2006;442(7101):448–52. doi: 10.1038/nature04795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Germann TC, Kadau K, Longini IM, Macken CA. Mitigation strategies for pandemic influenza in the United States. Proc Natl Acad Sci U S A. 2006;103(15):5935–40. doi: 10.1073/pnas.0601266103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halloran ME, Ferguson NM, Eubank S, Longini IM, Jr, Cummings DA, Lewis B, Xu S, Fraser C, Vullikanti A, Germann TC, et al. Modeling targeted layered containment of an influenza pandemic in the United States. Proc Natl Acad Sci U S A. 2008;105(12):4639–44. doi: 10.1073/pnas.0706849105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooley P, Brown S, Cajka J, Chasteen B, Ganapathi L, Grefenstette J, Hollingsworth CR, Lee BY, Levine B, Wheaton WD, et al. The role of subway travel in an influenza epidemic: a New York City simulation. J Urban Health: Bull New York Acad Med. 2011;88(5):982–95. doi: 10.1007/s11524-011-9603-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooley P, Lee BY, Brown S, Cajka J, Chasteen B, Ganapathi L, Stark JH, Wheaton WD, Wagener DK, Burke DS. Protecting health care workers: a pandemic simulation based on Allegheny County. Influenza Other Respir Viruses. 2010;4(2):61–72. doi: 10.1111/j.1750-2659.2009.00122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molinari NA, Ortega-Sanchez IR, Messonnier ML, Thompson WW, Wortley PM, Weintraub E, Bridges CB. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007;25(27):5086–96. doi: 10.1016/j.vaccine.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 27.FluVax View. [ http://www.cdc.gov/flu/fluvaxview/index.htm]
- 28.Physician Fee Schedule Search. [ https://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx]
- 29.Coleman MS, Fontanesi J, Meltzer MI, Shefer A, Fishbein DB, Bennett NM, Stryker D. Estimating medical practice expenses from administering adult influenza vaccinations. Vaccine. 2005;23(7):915–23. doi: 10.1016/j.vaccine.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 30.Pellissier JM, Coplan PM, Jackson LA, May J. The effect of additional shots on the vaccine administration process: results of a time-motion study in 2 settings. Am J Managed Care. 2000;6(9):1038–44. [PubMed] [Google Scholar]
- 31.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness–the curious resilience of the $50,000-per-QALY threshold. The New England J Med. 2014;371(9):796–7. doi: 10.1056/NEJMp1405158. [DOI] [PubMed] [Google Scholar]
- 32.Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA: J Am Med Assoc. 2016;316(10):1093–103. doi: 10.1001/jama.2016.12195. [DOI] [PubMed] [Google Scholar]
- 33.Chung JR, Flannery B, Thompson MG, Gaglani M, Jackson ML, Monto AS, Nowalk MP, Talbot HK, Treanor JJ, Belongia EA, et al. Seasonal effectiveness of live attenuated and inactivated influenza vaccine. Pediatrics. 2016;137(2):1–10. doi: 10.1542/peds.2015-3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zimmerman RK, Nowalk MP, Chung J, Jackson ML, Jackson LA, Petrie JG, Monto AS, McLean HQ, Belongia EA, Gaglani M. 2014–2015 Influenza vaccine effectiveness in the United States by vaccine type. Clin Infectious Dis. 2016;63(12):1564–73. doi: 10.1093/cid/ciw635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White PC, Baum DL, Ross H, Falletta L, Reed MD. Cocooning: influenza vaccine for parents and caregivers in an urban, pediatric medical home. Clin Pediatrics. 2010 doi: 10.1177/0009922810374353. [DOI] [PubMed] [Google Scholar]
- 36.Beel ER, Rench MA, Montesinos DP, Healy CM. Acceptability of immunization in adult contacts of infants: possibility of expanding platforms to increase adult vaccine uptake. Vaccine. 2014;32(22):2540–5. doi: 10.1016/j.vaccine.2014.03.056. [DOI] [PubMed] [Google Scholar]
- 37.Goodliffe L, Coleman BL, McGeer AJ. Wellness TDoOH, Safety: Acceptance of intradermal inactivated influenza vaccines among hospital staff following 2 seasonal vaccination campaigns. Hum Vaccines Immunotherapeutics. 2015;11(12):2827–30. doi: 10.1080/21645515.2015.1072665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flu Vaccination Coverage, United States, 2013–14 Influenza Season. [ http://www.cdc.gov/flu/fluvaxview/coverage-1314estimates.htm]
- 39.Alexandria VA, editor. 2014 Influenza vaccine production and distribution market brief. Health industry distributors association (HIDA); 2014. pp. 1–18. [Google Scholar]
- 40.Zimmerman RK, Lauderdale DS, Tan SM, Wagener DK. Prevalence of high-risk indications for influenza vaccine varies by age, race, and income. Vaccine. 2010;28(39):6470–7. doi: 10.1016/j.vaccine.2010.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.The medical letter on drugs and therapeutics: influenza vaccine for 2015–2016. The medical letter. 2015;57:125–7. [PubMed] [Google Scholar]
- 42.Glazner JE, Beaty B, Berman S. Cost of vaccine administration among pediatric practices. Pediatrics. 2009;124(Suppl 5):S492–498. doi: 10.1542/peds.2009-1542H. [DOI] [PubMed] [Google Scholar]
- 43.Lee BY, Bailey RR, Wiringa AE, Afriyie A, Wateska AR, Smith KJ, Zimmerman RK. Economics of employer-sponsored workplace vaccination to prevent pandemic and seasonal influenza. Vaccine. 2010;28(37):5952–9. doi: 10.1016/j.vaccine.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. The Lancet Infectious Dis. 2012;12(1):36–44. doi: 10.1016/S1473-3099(11)70295-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


