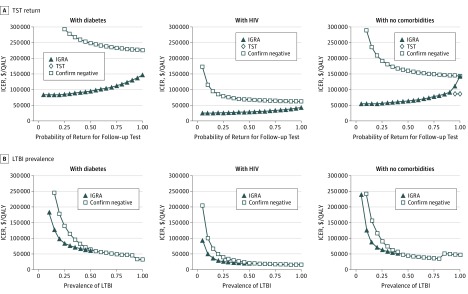
Figure 2. Effect of Tuberculin Skin Test (TST) Return and Latent Tuberculosis Infection (LTBI) Prevalence on Cost-effectiveness Conclusions in Non–US Born Patients.
A, A 1-way sensitivity analysis demonstrating the effect of TST return on the cost-effectiveness conclusions for interferon gamma release assay (IGRA) testing and IGRA plus TST for sensitivity testing. B, An illustration of the effect of LTBI prevalence on cost-effectiveness conclusions for IGRA testing and confirm negative testing. In the confirm negative strategy, patients first underwent IGRA. If that test was positive, LTBI was diagnosed. If that test was negative, then the patient underwent TST. LTBI was ruled out only if both tests were negative. For each risk population, there is a unique LTBI prevalence above which IGRA requires more investment to gain the same amount of quality-adjusted life-years (QALYs) than confirm negative. Above this point, IGRA is excluded from consideration as a viable strategy and thus is not represented in the figure. The apparent discontinuity at high prevalence (>90% non–US born patients with diabetes, >80% non–US born individuals with no comorbidities) emerges when confirm negative is not only a cost-effective strategy but becomes more favorable than other, less costly strategies. Incremental cost-effectiveness ratios (ICERs) are calculated against the next-best alternative strategy and are shown in 2015 US dollars per QALY gained. End-stage renal disease was excluded from this figure because it is cost-ineffective.