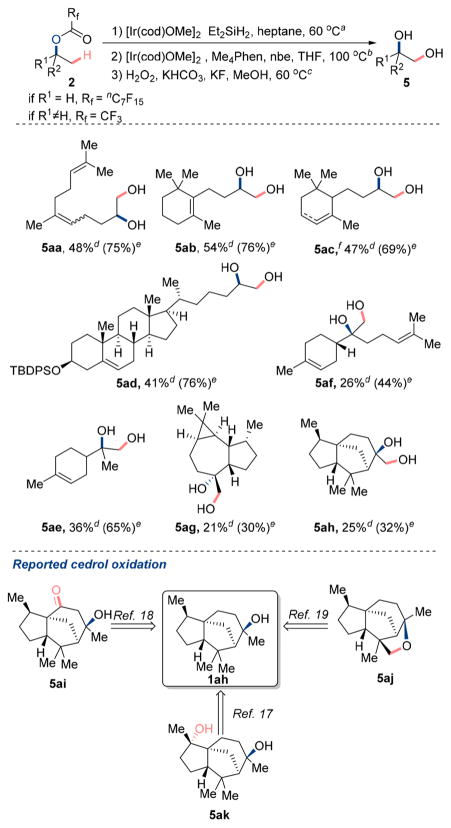
Scheme 5. Application of C–H Silylation/Oxidation Sequence to Natural Product Derivatives.
aHydrosilylation of ester: [Ir(cod)OMe]2 (1.0 mol %), Et2SiH2 (4.0 equiv), heptane (0.5 M), 60 °C, 48 h, N2. bC–H silylation step: [Ir(cod)OMe]2 (2.0 mol %), Me4Phen (6.0 mol %), nbe (1.5 equiv), THF (0.1 M), 100 °C, 16 h, N2. cOxidation step: KHCO3 (4.0 equiv), KF(4.0 equiv), 50% aqueous H2O2(10 equiv), MeOH, 60 °C, 2 h. dIsolated yield for the 1,2 diols over the three steps. eYield for the dioxasilinane determined by 1H NMR spectroscopy using CH2Br2 as internal standard. fStarting dihydro-α-ionone was purchased as the mixture of cyclohex-2-ene and cyclohex-3-ene derivatives in 4:1 ratio.