Table 1.
Evaluation of Fluorinated Directing Group
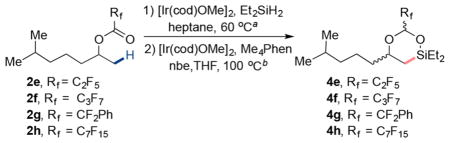
| |||
|---|---|---|---|
| entry | ester | conversionc | yieldd |
| 1 | 2d, Rf = CF3 | 100% | 45% |
| 2 | 2e, Rf = C2F5 | 100% | 68% |
| 3 | 2f, Rf = C3F7 | 96% | 78% |
| 4 | 2g, Rf = CF2Ph | 98% | 75% |
| 5 | 2h, Rf = C7F15 | 95% | 84%(75%)e |
Conditions for hydrosilylation of ester: [Ir(cod)OMe]2 (1.0 mol %), Et2SiH2 (4.0 equiv), heptane (0.5 M), 60 °C, 24–48 h, N2.
Conditions for β-C(sp3)–H silylation: [Ir(cod)OMe]2 (2.0 mol %), Me4Phen (6.0 mol %), nbe (1.5 equiv), THF (0.1 M), 100 °C, 16 h, N2.
Conversion for the hydrosilylation step determined by 1H NMR spectroscopy.
Overall yield for the two step determined by 1H NMR spectroscopy using CH2Br2 as internal standard.
Isolated yield.
