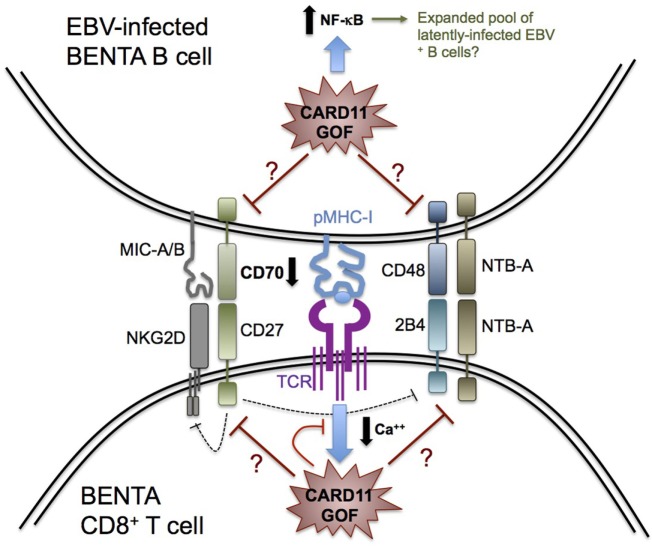
Figure 1.
Possible determinants of impaired Epstein–Barr virus (EBV) control in B-cell expansion with NF-κB and T-cell anergy (BENTA) disease. Schematic diagram summarizing key receptor–ligand interactions that govern CD8+ T cell recognition and killing of EBV-infected B cells, based on our knowledge of primary immune deficiencies featuring enhanced susceptibility to EBV-driven disease. CARD11 GOF signaling could perturb several molecular signals required for optimal cytolysis of EBV-infected B cells, including signaling lymphocyte activation molecule receptors (2B4, NTB-A), NKG2D, and CD27. For example, decreased CD70 expression on BENTA B cells could impair CD27 signaling and contribute to reduced NKG2D or 2B4 expression on BENTA T cells. Alternatively, attenuated TCR signaling (e.g., reduced Ca++ flux) likely contributes to BENTA patient T cell hyporesponsiveness, which could disrupt generation of CD8+ effector T cells with optimal cytotoxic function. Finally, elevated NF-κB signaling in B cells could accelerate the expansion of latently infected EBV+ B cells, contributing to detectable viremia as the virus continuously reactivates.