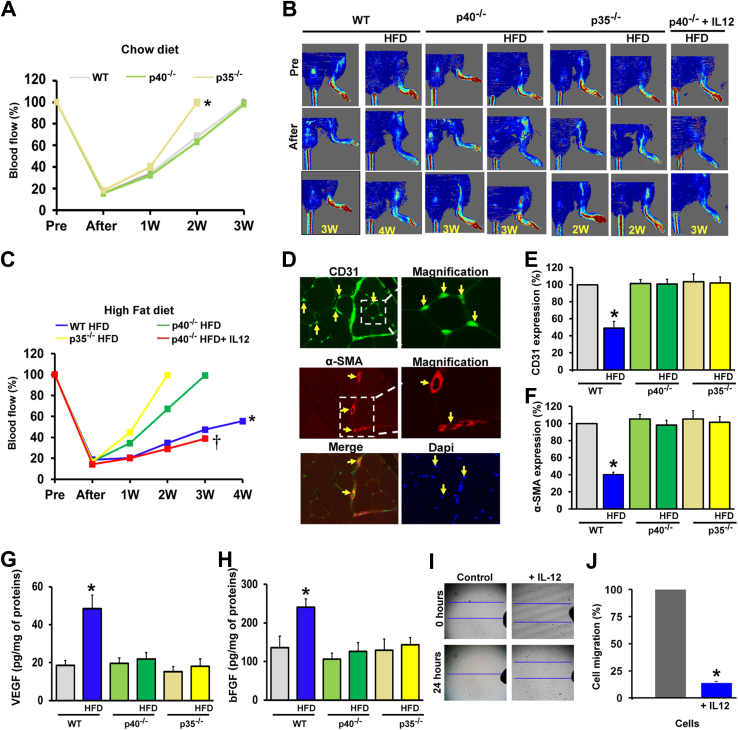
Figure 2.
Effect of IL-12 deficiency on ischemia-induced neovascularization in mice fed a normal diet or a high-fat diet (HFD). A and B: Quantitative data for blood flow recovery in wild-type (WT), p40−/−, and p35−/− mice fed a chow diet (A) or an HFD (B). C: Representative blood flow imaging measured with laser Doppler perfusion imaging (Moor Instruments) in the ischemic hind limb of WT, p40−/−, and p35−/− mice fed a chow diet or an HFD and p40−/− mice fed an HFD receiving exogenous IL-12 (25 ng, three times per week for 4 weeks) before surgery (Pre; femoral ligation), right after surgery, and once a week for 3 to 4 weeks (W). D: CD31 (capillaries; green), α-smooth muscle actin (α-SMA; arterioles; red) and DAPI (cell nuclei; blue) immunostaining of ischemic muscles in WT mice. The arrows show the specific staining. E and F: Cumulative data of CD31 and α-smooth muscle actin expression in the ischemic limb in WT, p40−/−, and p35−/− mice fed a chow diet or an HFD. G and H: Vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) levels measured by enzyme-linked immunosorbent assay in ischemic hind-limb muscle lysates from WT, p40−/−, and p35−/− mice fed a chow diet or an HFD. I and J: Effect of IL-12 on cell migration. Using the wound-healing assay, the wound was generated by a scratch on confluent vascular smooth muscle cells; the wound closure was monitored after scratch (0 hours) and at 24 hours and imaged with phase-contrast microscopy (Olympus, Lombard, IL). The migration assay was performed in three independent experiments. The P values were determined for control and IL-12–treated cells and P < 0.05 was considered statistically significant. Data are expressed as means ± SEM (E–H). n = 6 (C and E–H). ∗P < 0.05 versus all groups; †P < 0.05 versus p40−/− HFD group. Original magnification, ×10 (I).