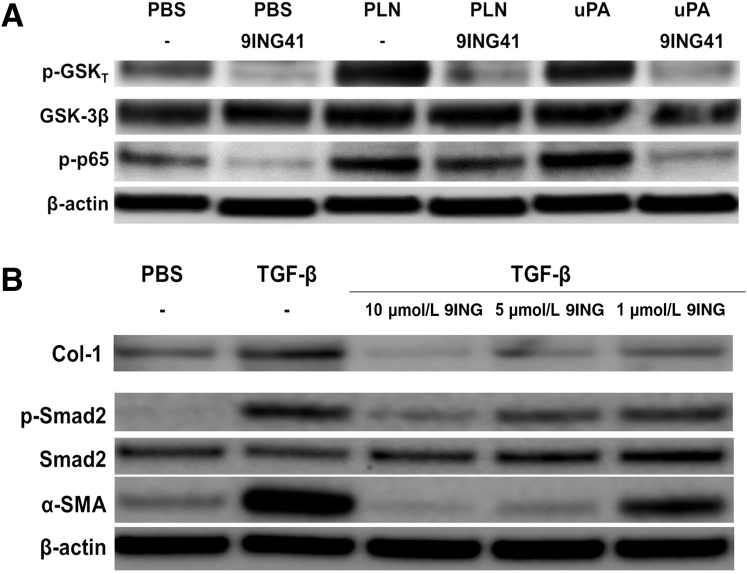
Figure 6.
Glycogen synthase kinase (GSK)-3β inhibition by 9ING41 reduces Tyr-216 phosphorylation of GSK-3β and phosphorylation of NF-κB and Smad2. A: Serum-starved human primary mesenchymal cells (HPMCs) were treated with phosphate-buffered saline (PBS), 7 nmol/L plasmin (PLN), and 20 nmol/L urokinase plasminogen activator (uPA) in the presence or absence of 10 μmol/L 9ING41. Cell lysates were then resolved by SDS-PAGE and immunoblotted for Tyr-216 phosphorylated GSK-3β (p-GSKT), total GSK-3β, and phosphorylated p65 (p-p65). β-Actin was used as loading control. B: Serum-starved HPMCs were treated with PBS and 5 ng/mL transforming growth factor (TGF)-β in the presence and absence of 9ING41 (10 to 1 μmol/L) for 48 hours. Conditioned media and cell lysates were then resolved by SDS-PAGE and immunoblotted for collagen (Col)-1, phosphorylated Smad2 (p-Smad2), total Smad2, and α-smooth muscle actin (α-SMA). β-Actin was used as loading control. n = 2 independent experiments.