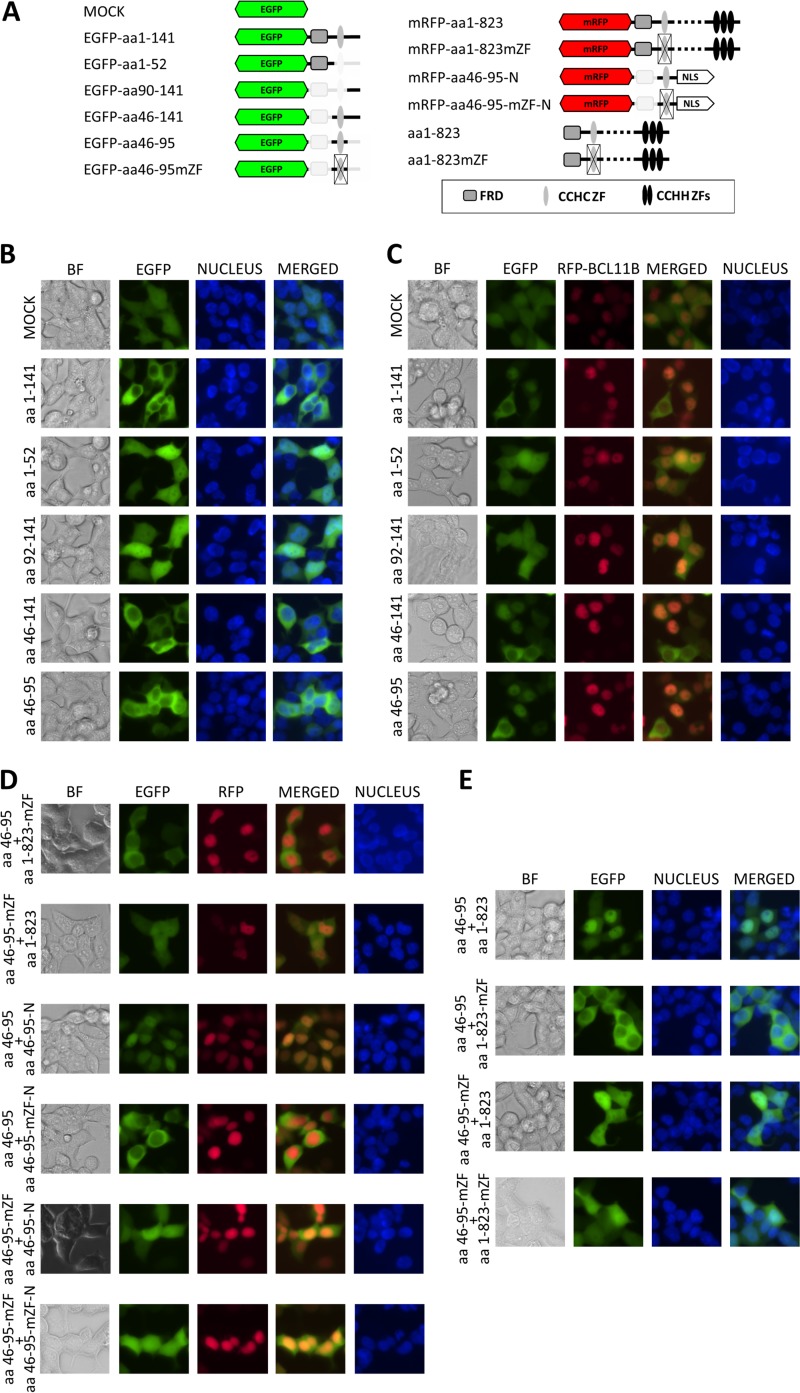
FIG 1.
The N-terminal part of BCL11B contains a dimerization domain. (A) Schematic drawing of BCL11B-derived EGFP and mRFP fusion peptides. (B) HEK293 cells were transfected with vectors encoding N-terminal fusions of EGFP and the indicated N-terminal fragments of BCL11B. The cells were visualized by fluorescence microscopy 24 h after transfection (original magnification, ×400). (C) The same fusion constructs as in panel B were cotransfected with mRFP-labeled full-length BCL11B. (D) Nonmutated CCHC motifs are required for nuclear uptake. (Top two rows) The CCHC domain fused to EGFP remains in the cytoplasm in the presence of full-length BCL11B containing a mutated CCHC ZF; EGFP fused to a mutated CCHC domain shows a diffuse pattern when cotransfected with wt BCL11B. (Bottom four rows) The mRFP-labeled and nucleus-directed (NLS-containing) CCHC motif transfers the EGFP-labeled CCHC peptides to the nucleus. Mutation of CCHC residues in at least one of the cotransfected fusion proteins abolished the colocalization. (E) The intact EGFP-tagged CCHC motif relocates to the nucleus in the presence of nonlabeled wt BCL11B but remains in the cytoplasm when coexpressed with a CCHC-mutated full-length variant. Impairment of CCHC in the EGFP-labeled construct results in a dispersed fluorescence pattern regardless of the CCHC status of the full-length BCL11B. The CCHC zinc finger structure was disordered by replacement of the zinc-coordinating residues with alanine (CCHC to AAAA). Nuclei were stained with the DNA-interacting stain Hoechst 33342. A representative of five independent experiments is shown. BF, bright-field microscopy.