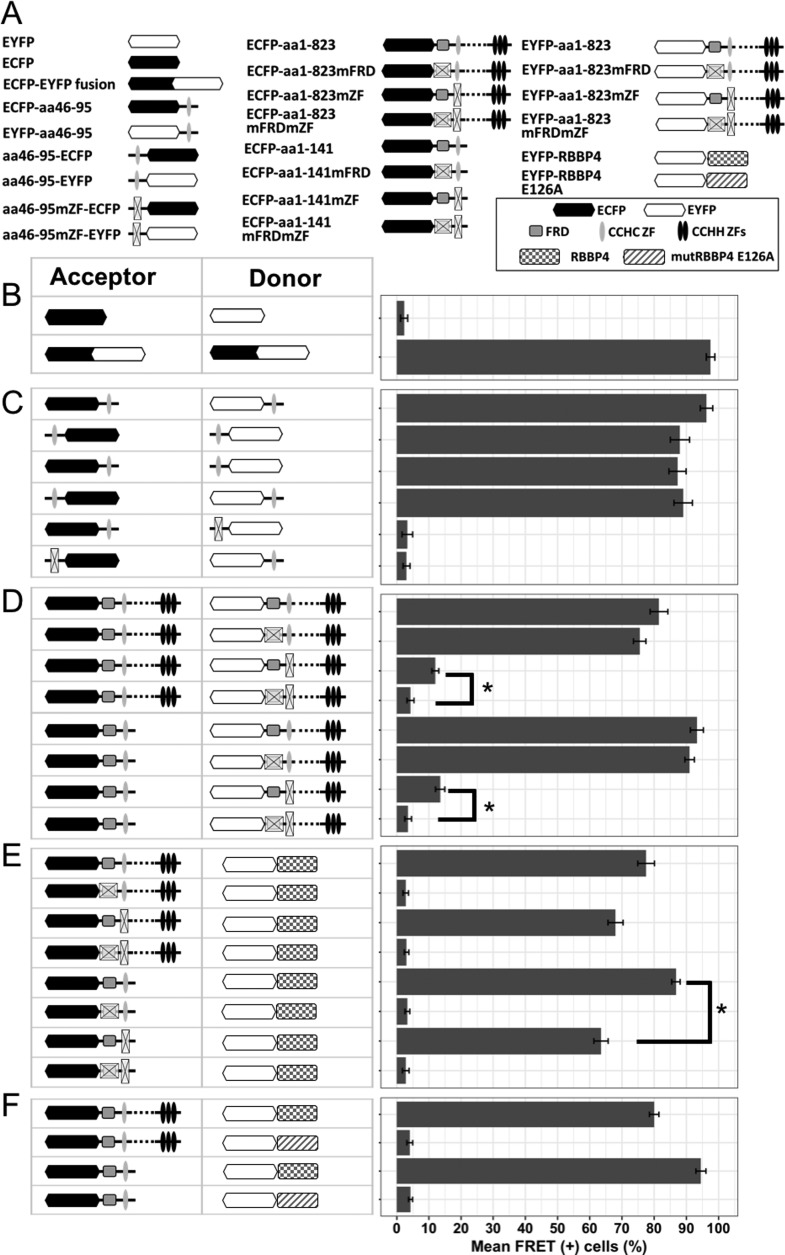
FIG 2.
Detection of direct protein-protein interactions by FACS-FRET assay. (A) Schematic representation of ECFP and EYFP fusions used for FRET assay. (B) HEK293 cells were transfected with either separate ECFP and EYFP FRET vectors (negative control) or a fused ECFP-EYFP construct (positive FRET control) and analyzed for FRET signal 24 h after transfection. (C) The isolated CCHC domain was linked with either ECFP or EYFP at its N or C terminus, and all possible combinations of labeled variants were cotransfected and checked for FRET signal. This excludes the position effect of the tags on the interaction. Mutation of the CCHC motif at the donor or acceptor site abrogated FRET. (D) The full-length BCL11B (top) or N-terminal deletion mutant (bottom) was used as a FRET donor, in combination with acceptor-labeled full-length BCL11B containing wt or mutated FRD and/or CCHC domains. (E) The coding sequence of RBBP4 fused with a FRET acceptor (EYFP) was cotransfected with full-length or N-terminal fragments of BCL11B tagged with ECFP, and variants with different statuses of FRD and/or CCHC were used as donors. (F) Wild-type RBBP4 or its mutant unable to bind the FOG-derived FRD domain (E126A) was fused to EYFP and cotransfected with ECFP-labeled full-length BCL11B or the fragment containing FRD and CCHC domains. The data represent mean values of four independent experiments with standard deviations (SD). *, P < 0,01.