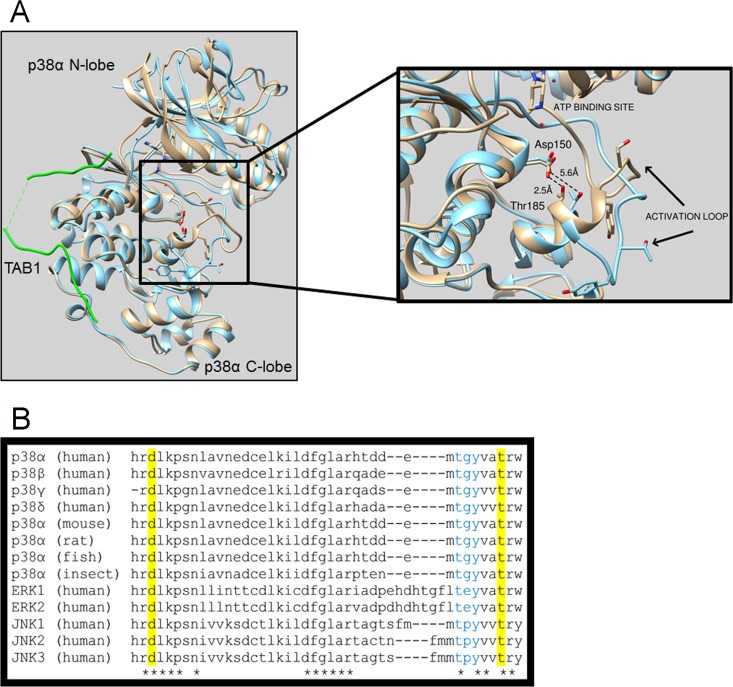
FIG 1.
(A) Superimposition of the crystal structures of free wtp38α (blue) and wtp38α (gray) in complex with TAB1 (green). In the free structure the side chain oxygens of Thr185 and Asp150 are separated by 5.6 Å, which prevents the formation of a hydrogen bond, and the activation loop is facing away from the catalytic site, a position not compatible with the autophosphorylation reaction. Upon TAB1 binding, Tyr182 to Thr185 form an alpha-helical segment that is stabilized by the hydrogen bond formed between Thr185 and Asp150 with side chain oxygens now 2.5 Å apart, typical of hydrogen bond formation. Consequently, the activation loop moves toward the catalytic site, bringing the T-G-Y motif in proximity to the γ-phosphate position of ATP, within its binding site, a position compatible with the autophosphorylation reaction. (B) Asp150 and Thr185 are conserved across p38 isoforms, species, and other mitogen-activated protein kinases.