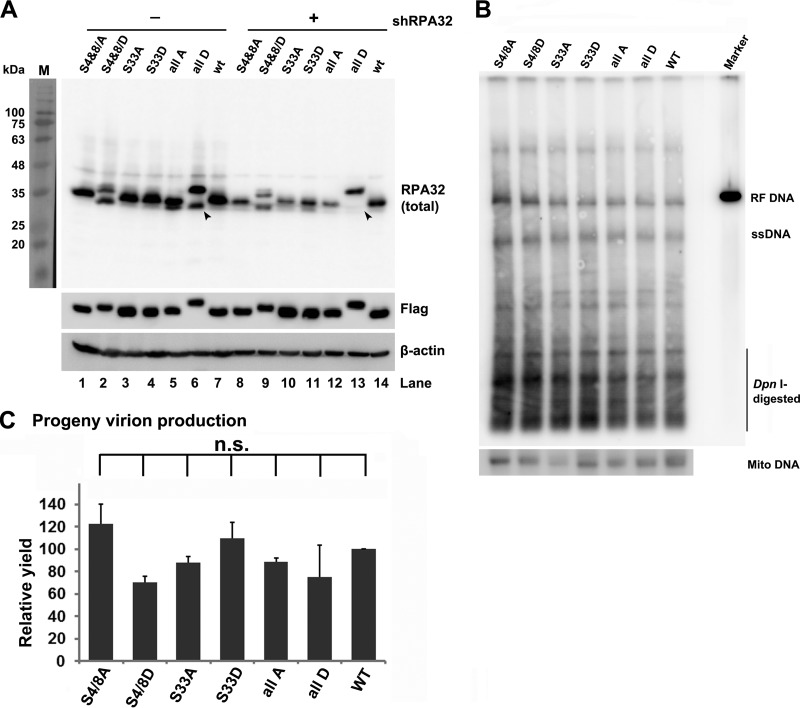
FIG 9.
Both phosphorylated and unphosphorylated RPA32 proteins play a role in B19V replication. (A) Expression of RPA32 mutants. Flag-tagged wild-type RPA32 and phosphorylation mutants, namely, RPA32(S4/8A, S4/8D, S33A, S33D, S4/8T21S33 to A or D mutations [all A or D]), were expressed in UT7/Epo-S1 cells by lentivirus transduction. Endogenous RPA32 was knocked down in these cell lines by transducing a shRPA32-expressing lentivirus. Protein expression of RPA32 mutants was detected using anti-RPA32 or anti-Flag antibodies. β-Actin was used as a loading control. Arrowheads indicate endogenous unphosphorylated RPA32. (B) Southern blot analysis of B19V DNA replication. RPA32 mutant-expressing UT7/Epo-S1 cells were cultured under hypoxia conditions and electroporated with SalI-linearized pM20. At 48 hpi, Hirt DNA was extracted from the transfected cells, digested with DpnI enzyme, and analyzed by Southern blotting using the M20 probe (upper panel) and the mitochondrial (Mito) DNA probe (lower panel). (C) B19V virion production. RPA32 mutant-expressing UT7/Epo-S1 cells were cultured under hypoxia conditions for 2 days and electroporated with SalI-linearized M20. At 48 hpi, the cell samples were collected and quantified for B19V virions using qPCR. The value for virus (vgc) produced from M20-transfected RPA32(WT)-expressing UT7/Epo-S1 cells was arbitrarily set at 100. n.s., not significant.