FIG 3.
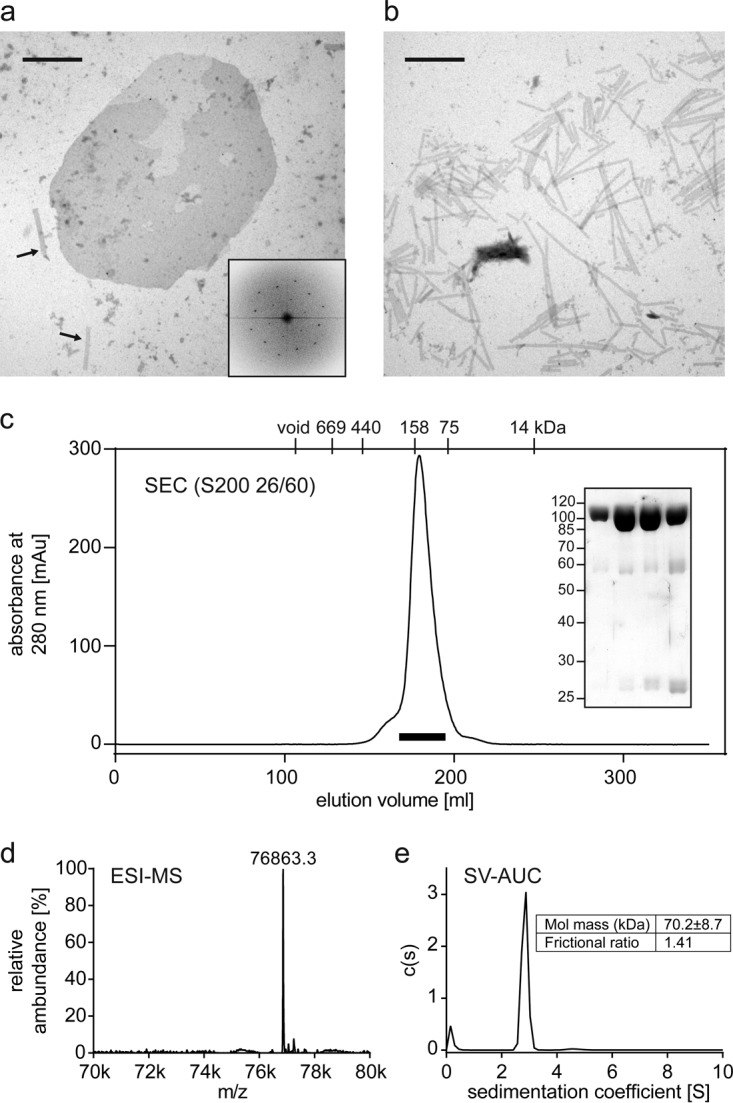
Production and biophysical characterization of the TMEA-cross-linked R82C E180D V181A capsid trimer. (a and b) Assemblies of HIV-1 capsid cysteine mutants that mimic the HIV-1 capsid surface and enable cross-linking of NTD-trimer interfaces. (a) Representative negative-staining EM image of in vitro-assembled R82C E180D V181A HIV-1 capsid. Assembly products form both elongated tubes, as seen on the bottom left (indicated by arrows), and planar sheets. One sheet is shown in the center of the micrograph. The scale bar corresponds to 1 μm. The hexagonal order of capsid molecules within an assembled HIV-1 capsid sheet is shown by a representative Fourier transform of a sheet image (inset). (b) Negative-staining EM image of in vitro-assembled R82C HIV-1 capsid lacking lattice-destabilizing mutations. This mutant forms elongated tubes only, as would be the case for the wild-type HIV-1 capsid (52). The scale bar corresponds to 1 μm. (c) Size exclusion chromatography (SEC) profile of the capsid trimer after disassembly of TMEA cross-linked R82C capsid lattices, with elution volumes of protein standards indicated on top of the panel. The SDS-PAGE analysis of 7-ml peak fractions indicated by the bar below the chromatography profile shows the high purity of the monodispersely eluting capsid trimer. The ESI-MS spectrum (d) and the sedimentation velocity analytical ultracentrifugation analysis (e) are consistent with the calculated mass of 76,866.3 Da for the HIV-1 capsid trimer. k, thousand.
