Fig. 4.
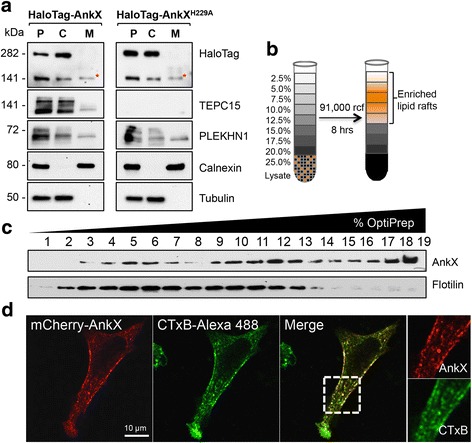
AnkX is present on membranes and co-localizes with lipid rafts. a Ectopically produced AnkX and endogenous PLEKHN1 localize to both the cytosolic and membrane fraction. HEK293T cells producing HaloTag-AnkX or -AnkXH229A were homogenized, and the post-nuclear supernatant (P) was separated by high speed centrifugation into the membrane (M) and cytosolic (C) fraction. HaloTag-AnkX was detected by immunoblot using anti-HaloTag antibody. The TEPC15 antibody was used to detect autophosphocholination of AnkX variants. Calnexin and tubulin served as marker for the membrane and cytosolic fraction, respectively. The asterisk indicates the membrane-associated HaloTag-AnkX that is shifted upwards relative to that in the cytosolic fraction. b AnkX co-localizes with Cholera Toxin subunit B, a major raft marker in biological membranes. Maximum intensity projection of transiently transfected HeLa cells producing mCherry-AnkX and labeled with Cholera Toxin subunit B conjugated to Alexa Fluor 488. Cells were fixed with 4% paraformaldehyde prior to imaging by confocal microscopy. c, d GFP-AnkX is present in fractions where lipid rafts are enriched. Transiently transfected HEK293T cells producing GFP-AnkX were lysed and subjected to detergent-free isolation of lipid rafts using OptiPrep discontinuous gradient centrifugation. The presence of GFP-AnkX in the fractions collected was determined by immunoblot using an anti-AnkX antibody. A Flotilin-2 antibody was used to reveal the fractions where lipid rafts were present. All immunofluorescence images and immunoblots are representatives of at least two independent experiments with similar outcomes. Scale bar: 10 μm
