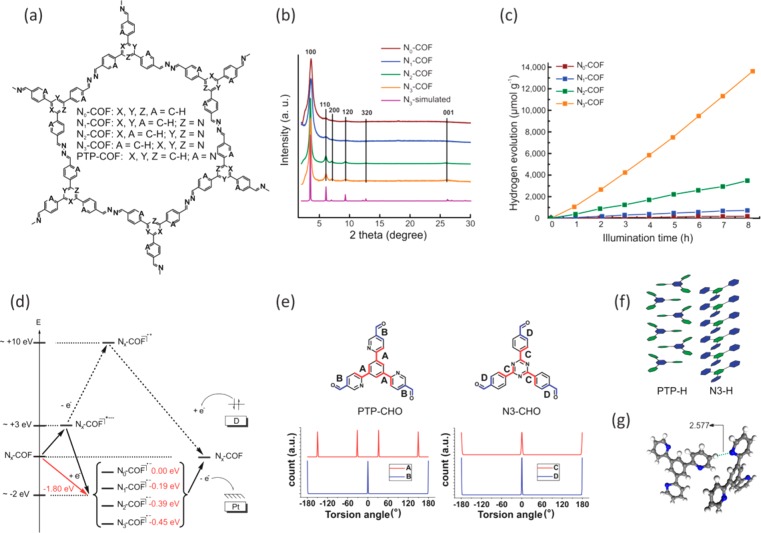
Figure 2.
Molecular structure (a) of the hexagonal pore of Nx- and PTP-COF. For the Nx-COFs, the crystallinity increases gradually from N0- to N3-COF as seen in the PXRD pattern (b). The H2 evolution rate with Pt cocatalyst and TEOA donor (c) analogously increases by 4 times for every additional N atom in the central aryl ring. The stability of the radical anion consonantly increases (d) as one goes from N0- to N3-COF. Four different conformations are possible around torsion angle A in PTP-CHO (e) as opposed to only two around torsion angle C in N3-CHO. Additional D–A type interactions (f) and H-bonding interactions (g) can be seen in single-crystal structure solutions of PTP-H. All of these possibly contribute to the lower crystallinity of PTP-COF. Panels a–d are adapted with permission from ref (40). Copyright 2015 by Nature Publishing Group. Panels e–g are adapted with permission from ref (41). Copyright 2017 Royal Society of Chemistry.