Table 2. Hydrogenation of N-containing substrates catalyzed by 10b a .
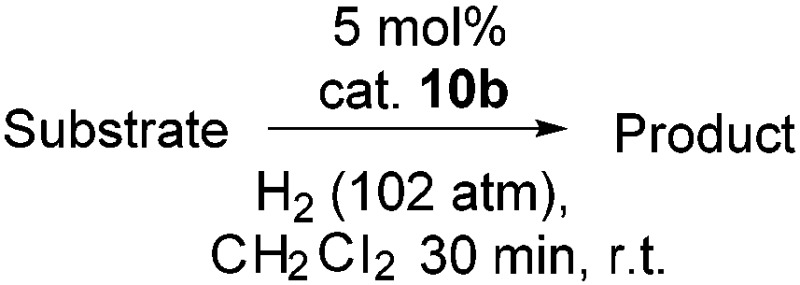
| |||
| Entry | Substrate | Product | Yield |
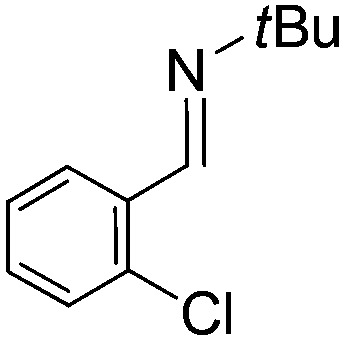
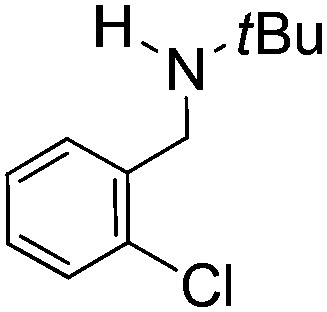
| 1 |
|
|
100 b (98) |
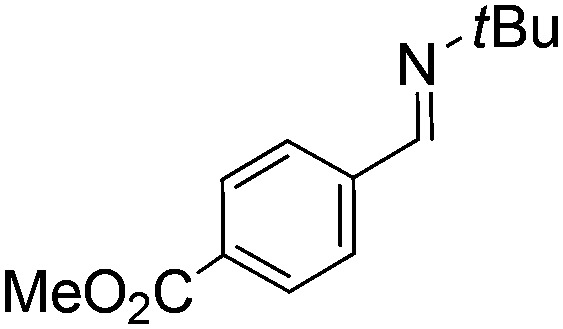
| 2 |
|
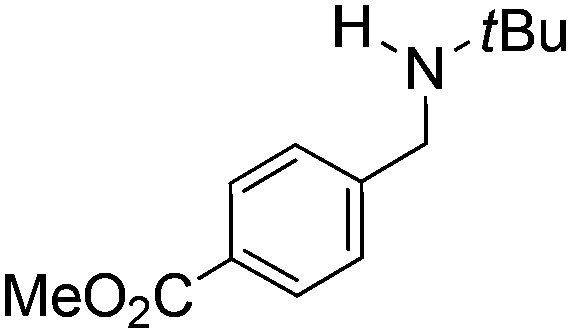
|
100 (82) |
| 3 |
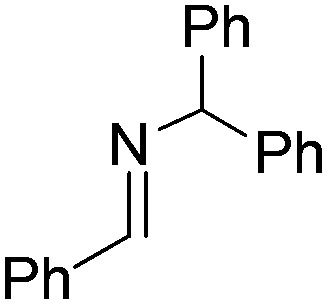
|
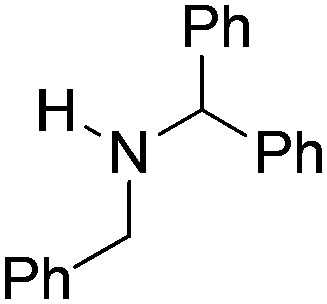
|
39 |
| 4 |
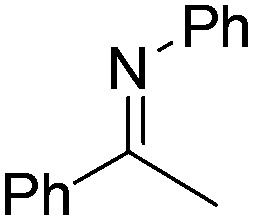
|
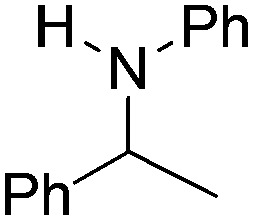
|
100 (71) |
| 5 |
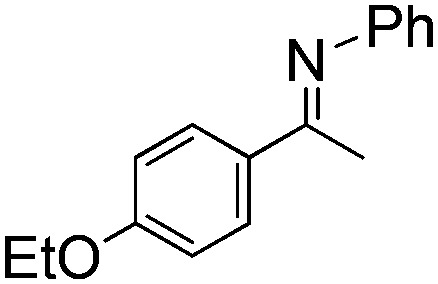
|
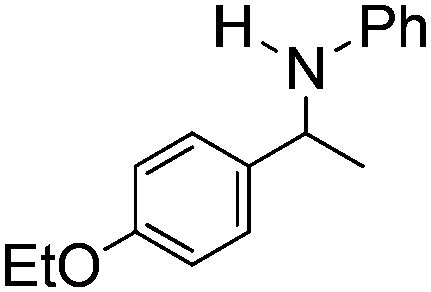
|
100 (95) |
| 6 |
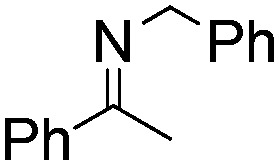
|
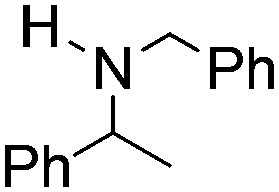
|
0 |
| 7 |
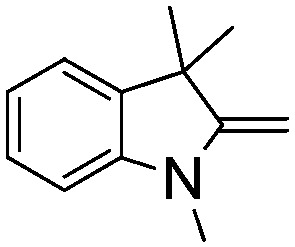
|
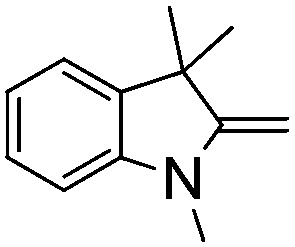
|
100 (91) |
| 8 |
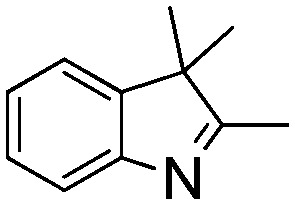
|
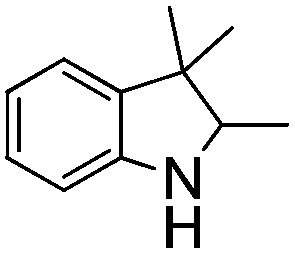
|
0 |
| 9 |
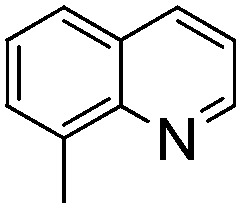
|
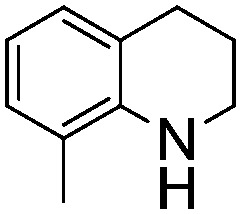
|
100 (87) |
aYields determined by 1H NMR spectroscopy, isolated yields in parentheses. All reactions were carried out using 0.500 mmol substrate in CH2Cl2. Reaction times were 30 minutes. Catalyst loadings: 5 mol% except:
b2.5 mol%.
