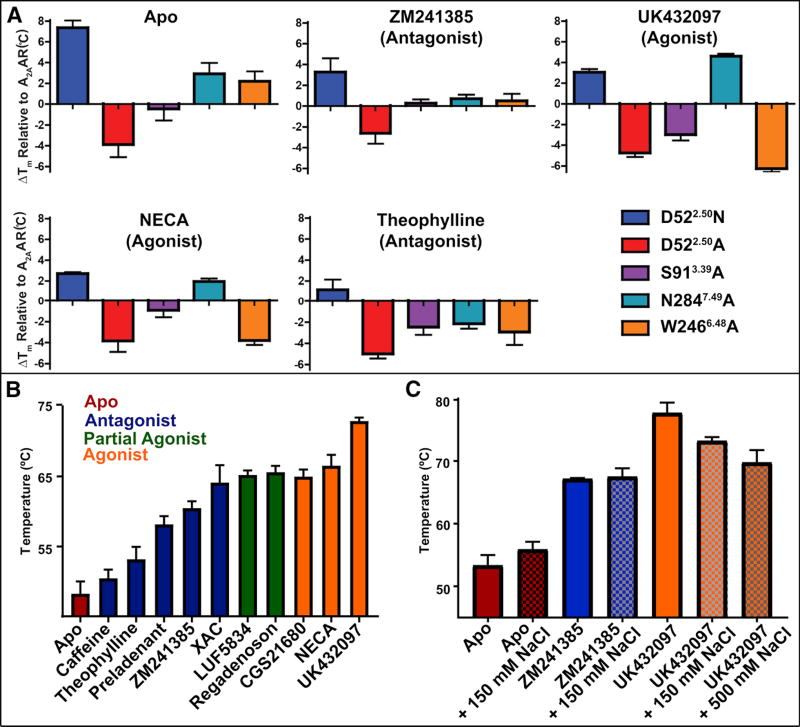
Figure 6. Thermal stability and function of A2AAR and allosteric site variants.
(a) Fluorescence thermal shift assays for A2AAR-BRIL and variants in complex with 5 different ligands and for no ligand added (apo). The mean changes in melting temperature (ΔTm) relative to A2AAR are displayed with error bars representing S.E.M. (n = 3) and performed at 150 mM NaCl. Tm values are listed in Table S1 and statistical analysis shown in Figure S5. (b) Tm values calculated from fluorescence thermal shift assays for A2AAR (without a fusion partner protein) for complexes with 10 ligands and apo. Ligands are identified below each column and the assay was performed at 75 mM NaCl. (c) Tm values for A2AAR-BRIL complexes with ligands in the absence (solid bars) and presence (checkered bars) of sodium (150 mM or 500 mM), as indicated. See also Figure S6.