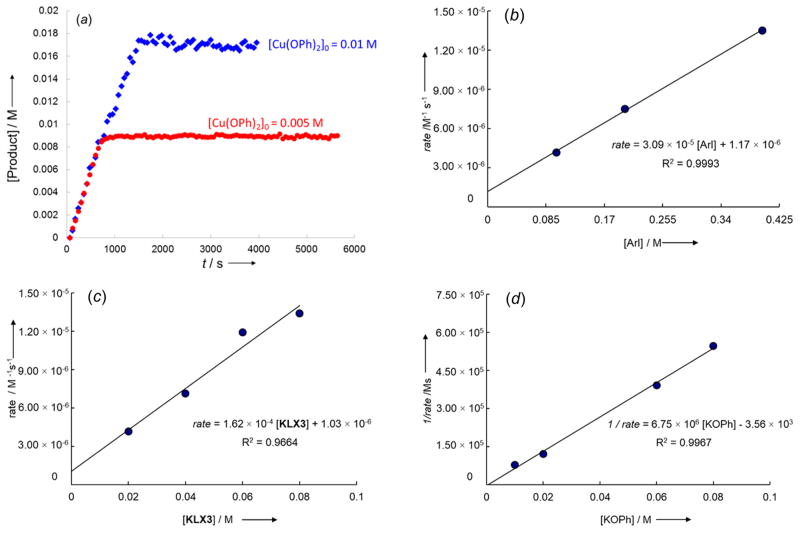
Figure 5.
(a) Plot of the rise of the biaryl ether product 2 from the reactions of p-iodotoluene (0.10 M) with 0.010 M (blue diamonds) or 0.005 M (red circles) of KCu(OPh)2 (1a) in the presence of KLX3 (0.080 M) in DMSO-d6 at 50 °C. (b) Plot of the [p-iodotoluene] vs the initial rate for the rise of the biaryl ether product 4 from the reaction of p-iodotoluene (0.10–0.40 M) with KCu(OPh)2 (1a, 0.01 M) in the presence of KLX3 (0.02 M) in DMSO-d6 at 50 °C. (c) Plot of the concentration of the ligand KLX3 ([L], 0.02–0.08 M) vs the initial rate for the rise of the biaryl ether product 2 from the reaction of p-iodotoluene (0.10 M) with KCu(OPh)2 (1a, 0.01 M) in DMSO-d6 at 50 °C. (d) Plot of the added [KOPh] (0.010–0.080 M) vs the reciprocal of the initial rate (1/rate) for the rise of the biaryl ether product 4 from the reaction of p-iodotoluene (0.20 M) with KCu(OPh)2 (1a, 0.01 M) in the presence of KLX3 (0.02 M) in DMSO-d6 at 50 °C.