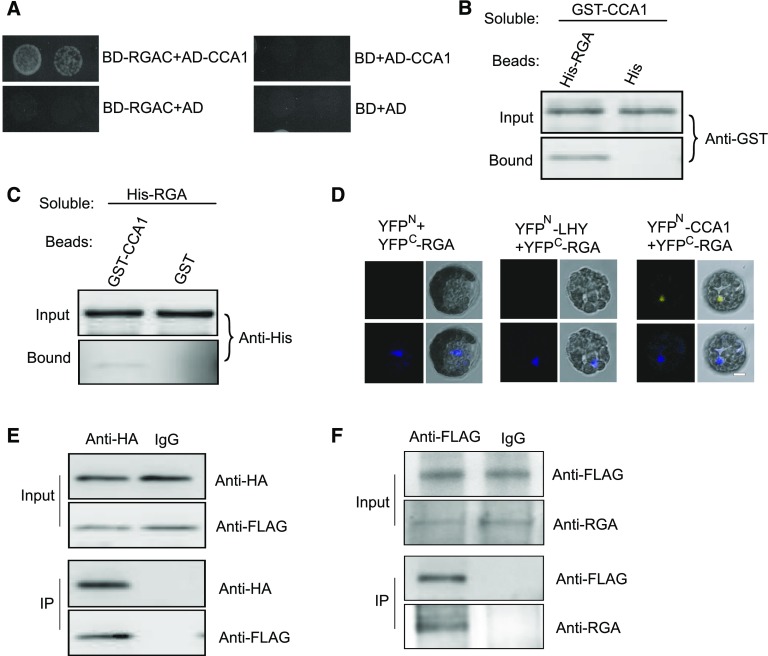
Figure 5.
RGA Interacts with CCA1.
(A) Yeast two-hybrid analysis revealing an interaction between the C terminus of RGA and CCA1. The growth of two concentrations (2 × 10−2 and 2 × 10−3) of yeast cultured on synthetic defined medium lacking Trp, Leu, His, and adenine is shown.
(B) Beads containing His tag (His) or His-fused full-length RGA were assayed for their ability to bind soluble GST-fused CCA1. The input and bound proteins were detected with an anti-GST antibody.
(C) Beads containing GST tag or GST-fused CCA1 were assessed for their ability to bind soluble His-fused RGA and detected with an anti-His antibody.
(D) CCA1 fused to the N terminus of YFP (YFPN) or LHY fused to the N terminus of YFP or the N terminus of YFP alone were tested for their ability to bind to the C terminus of YFP (YFPC) fused to full-length RGA. Yellow fluorescence and a bright field image were recorded and the resulting images were merged. Twenty-five cells were examined for each transformation. Bar = 10 µm.
(E) Co-IP between CCA1 and full-length RGA. FLAG-CCA1 and HA-RGA were cotransformed into Arabidopsis protoplasts, immunoprecipitated using an anti-HA antibody, and detected with anti-Flag and anti-HA antibodies. The cells were harvested at 2 h after lights-on zeitgeber time (ZT2).
(F) Co-IP of CCA1 and RGA in complemented plants. The cell extracts from 10-d-old seedlings were immunoprecipitated using an anti-FLAG antibody and detected with anti-FLAG and anti-RGA antibodies. The seedlings were harvested at 2 h after lights-on zeitgeber time (ZT2).