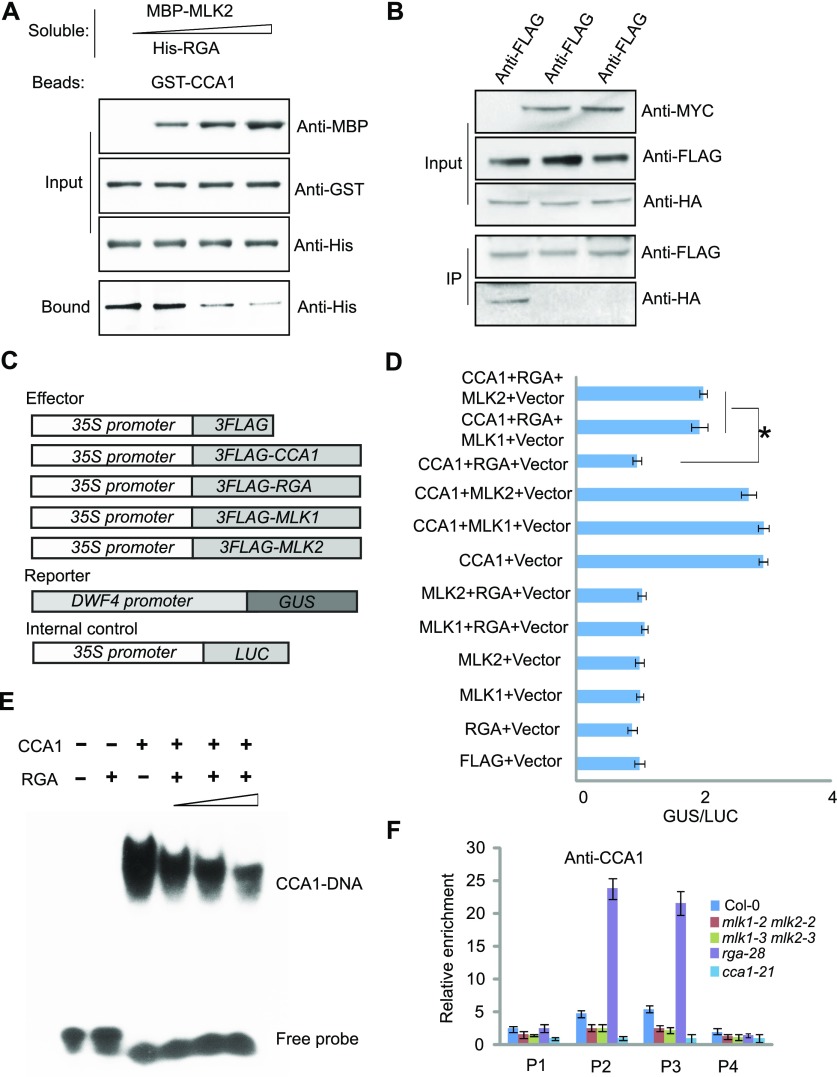
Figure 8.
MLK2 Antagonizes the Function of RGA to Bind CCA1 in Vitro and in Vivo.
(A) Competitive pull-down of CCA1 with RGA and MLK2. Beads containing GST-fused CCA1 were assayed for their ability to bind a soluble His-fused RGA with increased dosage of MBP-MLK2.
(B) Co-IP between RGA and CCA1 with or without MLK1/2. FLAG-CCA1 and HA-RGA were cotransformed with or without MYC-MLK1/2 into Arabidopsis protoplasts, immunoprecipitated using an anti-FLAG antibody, and then detected with anti-Flag and anti-HA. The cells were harvested at 2 h after lights-on zeitgeber time (ZT2).
(C) The vectors used in the GUS activity assay.
(D) The GUS activity from the ProDWF4:GUS reporter construct for cells transformed with CCA1, RGA, and MLK1/2. The x axis shows the relative GUS activity compared with the internal luciferase control (35S:LUC). Asterisks indicate P < 0.05 by t test.
(E) Gel shift assay with CCA1 and fragments of the DWF4 promoter region with increasing dosage of RGA. The binding ability of CCA1 to fragments of the DWF4 promoter labeled with 32P was assessed with increasing amounts of RGA (1, 4, and 10 µg).
(F) The relative enrichment of CCA1 at the DWF4 promoter was assessed using ChIP-PCR in rga28 and mlk1 mlk2 mutants. Experiments were repeated at least three times, and each experiment shown indicates the mean ± se, n = 3 replicates. The leaves were cross-linked at 2 h after lights-on zeitgeber time (ZT2).