Abstract
We reviewed emerging immune strategies for multiple myeloma (MM) therapy excluding US FDA approved drugs. In relapsed refractory MM, isatuximab (anti-CD38) monotherapy achieved overall response (OR) of 24%. Other monoclonal antibodies that have shown efficacy in combination therapy include siltuximab (OR: 66%), indatuximab (OR: 78%), isatuximab (OR: 64.5%), pembrolizumab (OR: 60%), bevacizumab (OR: 70%), dacetuzumab (OR: 39%) and lorvotuzumab (OR: 56.4%). No OR was observed with monotherapy using BI-505, siltuximab, bevacizumab, AVE-1642, figitumumab, atacicept, milatuzumab, dacetuzumab, lucatumumab, IPH2101, lorvotuzumab, BT062 and nivolumab. We included seven clinical trials on chimeric antigen receptor (CAR) T cells. CAR T-cell targets include BCMA, CD19, KLC and CD138. A recent experience of CAR T-cell (B-cell maturation antigen) therapy in advanced MM has shown global response of 100%. The future of monoclonal antibodies and adoptive T cells for MM treatment seems promising.
Keywords: : adoptive cell therapy, antibodies, antibody therapeutics, chimeric antigen T cells, immunotherapy, multiple myeloma
Multiple myeloma (MM) is characterized by malignant proliferation of plasma cells. It is the second most common hematologic malignancy and fourteenth leading cause of cancer deaths in the USA [1]. According to NIH cancer statistics, in 2017, there will be an estimated 30,280 new cases and 12,590 deaths due to MM [1]. Over the past one and a half decade, development of novel agents including proteasome inhibitors (PI) and immunomodulatory drugs (IMiDs) have improved disease outcome and overall survival (OS). According to survival data over 25 years, median survival of MM patients with International Staging System stages I and III has been reported 62 and 29 months, respectively [2]. Despite significant advancements in antimyeloma therapy, MM remains an incurable disease. Development of relapse and resistance is inevitable that signifies the need to develop newer treatment strategies [3]. Immunotherapy as a treatment modality for MM has the potential to be highly effective and targeted and is postulated to carry a favorable side effect profile. Various immunotherapeutic approaches are in use to treat malignant hematologic disorders including monoclonal antibodies (MoAbs), antibody–drug conjugate (ADC), bispecific antibodies and adoptive cell therapy (ACT).
Pathogenesis of MM is complex and involves heterogeneous cytogenetic abnormalities. The bone marrow microenvironment promotes cell growth and resistance to conventional therapies. Adhesion of MM cells to bone marrow stromal cells promotes proliferation of malignant cells, production of anti-apoptotic signals, angiogenesis, bone resorption and tissue invasion. Pathogenesis involves various growth factors, cytokines, surface receptors and intracellular signaling molecules [4]. Potential immunotherapy targets for MM are cell surface molecules including adhesion molecules and transmembrane receptors for intracellular signaling such as CS1, CD38, CD138, CD40, CD47, CD74, CD54, CD56, CD19, IGF-1R, kappa light chain and BCMA). Targets also include growth factors and their receptors like VEGF and BAFF. Other targets include IL-6 and immune checkpoint signaling pathways using programmed death (PD-1 axis) [5,6]. MoAbs exert their antitumor effects through several mechanisms. Antibody-dependent cellular cytotoxicity (ADCC) occurs when MoAb binds to tumor cells through their hypervariable region and immune cells through the Fc region triggering cell death. Complement-dependent cytotoxicity is a mechanism in which therapeutic antibodies activate proteolytic enzymes ultimately resulting in the formation of terminal lytic complex that attacks tumor cell membrane [7]. Alternatively, MoAb can act as an agonist to trigger apoptosis. Therapeutic antibodies can act as antagonists on cell surface receptors to block downstream signaling involved in cell proliferation. In ADCs antibodies serve as carriers to deliver cytotoxic drugs specifically inside tumor cells to exert their maximum antitumor effects while preventing their systemic adverse effects [8]. Elotuzumab and daratumumab are already approved for the treatment of MM and are currently under evaluation in phase III trials.
Adopive cell therapy involves modification of autologous T cells or NK cells to express chimeric antigen receptors (CARs) that bind specifically to antigens expressed on tumor cells. CAR-modified T cells (CAR T cells) targeting CD19 have been used in treatment of chronic lymphocytic leukemia and acute lymphoblastic leukemia, which stimulated interest in the extension of ACT for treatment of MM. An ideal target for CAR T-cell therapy would be one that is exclusively expressed on MM cells to avoid systemic toxicity while playing a central role in tumorigenicity to restrict antigen escape. Several strategies are under evaluation to minimize systemic toxicity from CAR T-cell therapy. The suicide gene strategy stimulates the death of CAR T cells in the event of any toxicity [9]. An alternative strategy utilizes co-inhibitory receptors that are only expressed on healthy tissues and not on tumor cells. In the event of toxicity these receptors would inhibit CAR T cell effect on healthy tissues[10]. Another strategy is to minimize trafficking of CAR T cells to non-tumor sites by genetically engineering CAR T cells to express specific chemokine receptors [10]. Our aim is to summarize the current state of knowledge on the efficacy of non-US FDA approved MoAbs, CAR T cells and their targets.
Methods
The systematic review was designed in accordance with the principles set by the preferred reporting items for systematic reviews and meta-analyses (PRISMA) checklist.
Search strategy
Databases searched for English language studies included PubMed, Cochrane, EMBASE, Web of Science and Clinicaltrilas.gov. For clinicaltrials.gov, only completed studies or those actively recruiting were included. In addition to English language filter, only studies from last 10 years were included.
A sample search strategy is given below: “Multiple myeloma”[MeSH Terms] AND “recombinant fusion proteins”[MeSH Terms] AND (“2007/07/05”[PDat] : “2017/07/01”[PDat] AND English[lang]).
To avoid missing any potentially relevant studies, bibliographies of pertinent review articles were analyzed manually.
Eligibility criteria
We included clinical trials on MoAb for treatment of MM that were complete or actively recruiting Phase I/II trials performed in the last 10 years with at least one efficacy outcome clearly described in both treatment and control arm (if any). We excluded studies on daratumumab and elotuzumab. We included clinical trials on CAR T cells that were Phase I/II clinical trials focusing on CAR T cells in relapsed and refractory MM (RRMM) with at least one efficacy outcome clearly described. We excluded studies in which no objective efficacy outcome was reported.
Study selection & data extraction
Articles retrieved using aforementioned search strategy were imported to endnote. Articles were screened first based on title and abstract and then through full text by two independent reviewers. A standardized data extraction form was used to extract the fields: study, year, study design, number of patients, MoAb and its target, median number of prior lines of therapy, dose, number of cycles, regimen used, lymphodepletion therapy used before CAR T-cell infusion, targeted antigen and its location, outcome and adverse effects. Primary outcome was overall response (OR). Other outcomes were complete response (CR), partial response (PR), stable disease (SD), progressive disease (PD), progression-free survival (PFS) and OS. The toxicity data were analyzed for cytokine-release syndrome, common adverse effects and Grade ≥III adverse effects. Any conflicts during the process of study selection and data extraction were resolved with discussion and consensus.
Results
Monoclonal antibodies
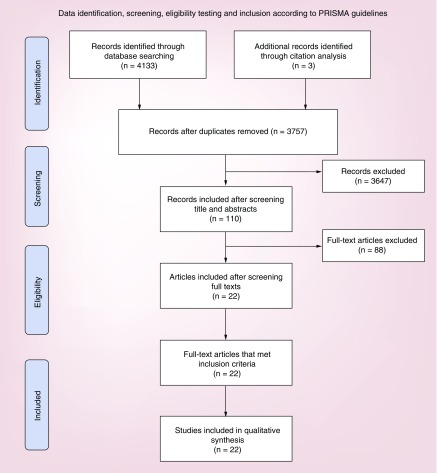
In total, we identified 4133 articles from the following resources: PubMed (n = 81), Embase (n = 929), Cochrane (n = 21), SCOPUS (n = 2000), Web of Science (n = 1481) and clinicaltrials.gov (n = 47). After excluding 379 duplicates, we first screened articles by reading titles and abstracts. Later, potentially relevant articles were screened through full texts (Figure 1). We excluded articles related to drugs that are already FDA approved as daratumumab or elotuzumab in RRMM. 22 clinical trials that met the inclusion criteria included a total of 1130 patients. Totally, 5 studies had control arms and 17 were single arm studies.
Figure 1. . Records identified through PubMed, Embase, Cochrane, Web of Science, Scopus and ClinicalTrials.gov database searches.
We evaluated following antibodies (n = number of studies): bevacizumab (anti-VEGF; n = 2), dacetuzumab (anti-CD40; n = 2), lucatumumab (anti-CD40; n = 1), isatuximab (ISA) (anti-CD38; n = 3), siltuximab (anti-IL-6; n = 3), tabalumab (anti-BAFF; n = 2), lorvotuzumab (anti-CD56; n = 2), indatuximab (anti-CD138; n = 3), rituximab (anti-CD20; n = 1), nivolumab (anti-PD-1; n = 1), pembrolizumab (anti-PD-1; n = 1) and BI-505 (anti-ICAM-1; n = 1).
MoAb targets included ICAM-I (BI-505) = 1 study, IL-6 (siltuximab) = 5, VEGF (bevacizumab) = 4, GRP78 (PAT-SMX), CD40 (dacetuzumab, lucatumumab) = 4, CD138 (indatuximab) = 3, PD-1 (pembrolizumab, nivolumab, pidilizumab) = 3, CD20 (rituximab), CD38 (ISA), CD56 (lorvotuzumab) = 3, IGF-1R = 3, (AVE1642, figitumumab), BAFF (atacicept, tabalumab) = 3 and CD74 (milatuzumab) = 1 study.
Surface receptor-targeting antibodies
Anti-CD38 MoAb
CD38 is a single chain transmembrane molecule that is expressed on cell surface and intracellular organelles of terminally differentiated B lymphocytes. It acts as a receptor to activate the proliferation of T cells via cytokine release and cell-to-cell adhesion and has a catalytic activity during synthesis of cyclic adenosine diphosphate ribose [5]. ISA is an anti-CD38 MoAb that produces its antitumor effects by ADCC, complement-dependent cytotoxicity or direct toxicity. Two trials with ISA as monotherapy by Martin et al. [11] and Richter et al. [12] have reported objective response rate (ORR) of 32 and 24% in patients with six and five prior lines of therapy, respectively. When ISA was used in combination with lenalidomide and dexamethasone (Dex), this combination produced an ORR of 65% in heavily pretreated RRMM patients with six prior lines of therapy [13]. With 10 mg/kg of ISA, no dose-limiting toxicity was reported. However, in Martin et al. study, grade III pneumonia, fever, hyperglycemia and hypophosphatemia were reported with higher dose of 20 mg/kg of ISA. After promising Phase I data, a randomized prospective multicenter Phase III trial (open-label study: NCT02990338; ICARIA-MM) to evaluate the clinical benefit of ISA in combination with pomalidomide (Pom) and low-dose Dex (ISA/Pom/Dex) versus Pom/Dex is ongoing [14,15]. Currently ISA is being tested in doses of 5, 10 or 20 mg/kg weekly for the first cycle and then every 2 weeks for subsequent cycles (Table 1).
Table 1. . Surface receptor-targeting antibodies in relapsed refractory multiple myeloma.
| Study (year) study design | Number of patients | Antibody | Regimen | Target | Median prior therapies | Dose | Number of cycles | Outcome | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Vij et al.(2016) Phase II | 97 | Isatuximab SAR650984 | Isatuximab monotherapy | CD38 | 5 (2–14) | 3–10 mg/kg | NR | OR = 24% at dose >10 mg/kg | [12] |
| Martin et al. (2014) Phase Ib | 31 | Isatuximab SAR650984 | Isatuximab + Len + Dex | CD38 | 6 (2–12) | 3, 5, 10 mg/kg | NR | OR = 64.5%; CR = 76.8%; VGPR = 26%; PR = 32%; PFS = 6.2 m | [13] |
| Martin et al. (2014) Phase I | 35 | Isatuximab (SAR650984) | MoAb monotherapy | CD38 | 6 (2–14) | 0.1–20 mg/kg | 5–7 | OR = 32% (>10 mg); PR: n = 6; CR: n = 2 | [11] |
| Kaufman et al. (2013) Phase I | 25 | Milatuzumab | Monotherapy | CD74 | 5 | 1.5–16 mg/kg ×2 or 4 weeks | 8 | No OR; SD = 26% (5/19) >3 m; (1/19)>17 m | [16] |
| Moreau et al. (2011) Phase I | 11 | AVE1642 | AVE1642 + bortezomib | IGF-1 | 4 | 0.5–12 mg/kg + 1.3 mg/m2 | 4 | CR: n = 1; PR: n = 1; SD: n = 3 | [17] |
| 15 | AVE1642 | AVE1642 monotherapy | IGF-1 | 4 | 3–18 mg/kg | 2 | MR: n = 1; SD: n = 7; PD: n = 4 | ||
| Lacy et al. (2008) Phase I | 47 | Figitumumab (CP: 751, 871) | Figitumumab + Dex if no PR on figitumumab monotherapy | IGF-1 | 4 (0–8) | 0.025–20 mg/kg for 4 weeks | 4 | No OR | [18] |
| 27 | Figitumumab (CP: 751, 871) | Figitumumab + Dex | IGF-1 | 4 (0–8) | 0.025–20 mg/kg for 4 weeks + 40 mg/day Dex | PR: n = 6 | |||
| Benson et al. (2015) Phase I | 15 | IPH 2101 | IPH 2101 + Len (10–25 mg) | KIR | 1–2 | 0.2–2 mg/kg | 4 | VGPR = 13%; PR = 20%; MR = 7%; SD = 40%; PD = 20% | [19] |
| Benson et al. (2012) Phase I | 32 | IPH 2101 | Monotherapy | KIR | 2 (1–7) | 0.0003–3 mg/kg every 28 days | 4 | No OR; SD: n = 11 (34%) | [20] |
| Agura et al. (2009) Phase Ib | 36 (33 REP) | Dacetuzumab | Dacetuzumab + Len + Dex | CD40 | 4 | 4–12 mg/kg | 4 | OR = 39%; CR = 3%; PR = 33%; MR = 12%; SD = 30%; PD = 6%; NE = 12% | [21] |
| Hussein et al. (2010) Phase I | 44 | Dacetuzumab | MoAb monotherapy | CD40 | 5 (2–14) | 4–12 mg/kg | 4–5 | SD = 20% | [22] |
| Bensinger et al. (2012) Phase I | 28 | Lucatumumab | MoAb monotherapy | CD40 | NR | 1–6 mg/kg | 4 | SD = 43%; PR = 4% >8 m | [23] |
| Hansson et al. (2015) Phase I | 35 (29 REP) | BI-505 | MoAb monotherapy | ICAM-1 | 6 | 0.0004–20 mg/kg | 1–2 | SD = 24% (2 m); PD = 65% | [24] |
CR: Complete response; Dex: Dexamethasone; ICAM-1: Intercellular adhesion molecule-1; KIR: Killer-cell immunoglobulin like receptor; Len: Lenalidomide; m: Month; MoAb: Monoclonal antibody; MR: Minimal response; NE: Not evaluable; NR: Not reported; OR: Objective response; PD: Progressive disease; PFS: Progression-free survival; PR: Partial response; REP: Response evaluable patient; SD: Stable disease; VGPR: Very good partial response.
Anti-CD74 MoAb
CD74 is a membrane-associated glycoprotein and is present in B cells, monocytes and myeloma cells. CD74 is important for MHC II trafficking and antigen loading. CD74 also has a particular importance because of its rapid internalization [25]. This property would help deliver cytotoxic agents inside the cells and a trial with anti-CD74 humanized MoAb milatuzumab combined with doxorubicin is evaluating the potential for its therapeutic target. In a Phase I multicenter clinical trial, 25 patients with advanced MM were treated with milatuzumab infusion that was given twice weekly for 4 weeks. Totally, 19 patients completed treatment. No OR was achieved but 5/19 patients had SD 3 months post-treatment, one patient had SD for 17 months, 20% patients experienced cytokine release syndrome (CRS) [16]. Current evidence supports the need for further testing of milatuzumab in combination chemotherapy trials (Table 1).
Anti-IGF-1 receptor antibody
IGF-1 receptor and binding proteins are involved in tumor survival, proliferation, angiogenesis and osteolysis and play a crucial part in the pathogenesis of MM. The increased expression of IGF-1 in MM cells correlates with poor outcome and resistance to standard treatment [26]. Anti-IGF-1 receptor MoAbs (AVE1642 and figitumumab) have been tested in Phase I trials as monotherapies versus in combination with Dex or bortezomib. When AVE1642 was used as monotherapy, no ORR was achieved. However, when AVE1642 was used in combination with bortezomib, this combination produced PR in 2 out of 11 patients [17]. Figitumumab in combination with Dex produced PR in 6 out of 27 patients [18]. Despite good safety profile, these responses are not considered clinically sufficient (Table 1).
Antikiller-cell immunoglobulin-like receptor antibody
Killer-cell immunoglobulin-like receptor (KIR) are transmembrane glycoproteins expressed on surface of NK cells. Most KIRs are inhibitory, in other words, they suppress cytotoxic activity of NK cells. IPH-2101 is an anti-KIR MoAb that augments antitumor activity of NK cells. However, this effect was limited when IPH-2101 was used as monotherapy in in vivo studies due to antibody-mediated hyporesponsiveness and contraction of NK cell subset [27]. As monotherapy, IPH2101 did not produce any objective response [20]. In a Phase I trial, IPH-2101 was used in combination with lenalidomide in 15 patients with RRMM. Dose ranging from 0.2–2 mg/kg was used on day 1 of 21–28 day cycle. An OR of 33.3% (n = 5) was achieved: two very good partial response (VGPR) and three PR. Six patients had stable disease (SD). This drug combination was well tolerated [19] but use of IPH-2101 in smoldering MM as monotherapy in a Phase I–II trial failed to produce any clinical response (Table 1) [28].
Anti-CD40 MoAb
CD40 is a surface receptor of the TNF family and is expressed in B-cell malignancies including MM [29]. Dacetuzumab is the first anti-CD40 MoAb that was tested as monotherapy and in combination with lenalidomide and Dex. As monotherapy, it was tested in 44 RRMM patients with 5 prior lines of therapy with maximum tolerated dose of 12 mg/kg. No OR was achieved. Totally, 20% patients had SD [22]. When used in combination with lenalidomide and Dex in a population of 36 RRMM patients with four prior lines of therapy, dacetuzumab produced an OR of 39% [21].
Lucatumumab is also an anti-CD40 MoAb that has a dual mechanism of action. It blocks CD40-CD40L dependent tumor growth and induces cell lysis via ADCC [23]. Lucatumumab was tested as monotherapy in 28 RRMM patients with 3 prior lines of therapy. Only one patient achieved PR while 43% had SD (Table 1) [23].
Anti-ICAM-1 (CD54) MoAb
ICAM-1 plays an important role in adhesion of myeloma cells to marrow stromal cells and helps in tumor proliferation. Overexpression of ICAM-1 has been associated with chemotherapy-resistant advanced disease [30]. BI-505 is fully humanized IgG1 MoAb that targets ICAM-1. In preclinical studies, BI-505 has shown potent antimyeloma activity [31]. However, when tested as monotherapy in patients with RRMM (6 prior lines of therapy) and in smoldering MM patients, no OR was achieved (Table 1) [24,32].
Nonsurface target antibodies
Anti-IL-6 antibody
IL-6 is a pleiotropic cytokine that plays a vital role in T- and B-cell immune responses. It is a potent mediator of inflammation, regulates hematopoiesis, and is involved in proliferation and differentiation of malignant plasma cells. Siltuximab is IL-6 blocking antibody that has already shown safety and efficacy in Castleman disease [33]. Preclinical studies of siltuximab showed significant anti-MM activity when used in combination of bortezomib and Dex [34,35].
For RRMM, there have been three trials with siltuximab by Orlowski et al., Voorhees et al. and Suzuki et al. Orlowski et al. in a head-to-head comparison of siltuximab and bortezomib versus bortezomib alone found no statistically significant difference in ORR (55 vs 47%), median PFS (8 vs 7.6 months) and OS (30.8 vs 36.8 months), respectively. Their patient population had 1–3 prior lines of therapy [36]. Voorhees et al. compared activity of siltuximab alone versus siltuximab and Dex in patients with four prior lines of therapy and found no OR with siltuximab monotherapy. In combination with Dex, they reported an ORR of 23% [37]. Suzuki et al. studied siltuximab in combination with lenalidomide and Dex in nine RRMM patients with 1–2 prior lines of therapy. They reported CR of 22% and PR of 44% in their study [38].
For newly diagnosed MM, we summarized data from two trials by San-Miguel et al. and Shah et al. San-Miguel et al. performed head-to-head comparison of bortezomib, melphalan and prednisone (VMP) (n = 54) versus VMP + siltuximab (n = 52). The study did not reach the primary outcome that the addition of siltuximab to VMP regimen will increase CR by at least 10%. The CR for siltuximab + VMP and for VMP alone was 27 and 22%, respectively, without reported improvement in long-term outcomes [39]. Shah et al. studied siltuximab + RVd (lenalidomide, bortezomib and dexamethasone) in 11 newly diagnosed MM patients and reported an impressive ORR of 91% (Table 2) [40].
Table 2. . Nonsurface receptor antibodies in relapsed refractory multiple myeloma.
| Study (year) study design | Number of patients | Antibody | Target | Median prior therapies | Dose | Number of cycles | Regimen | Outcome | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Suzuki et al. (2015) Phase I | 9 | Siltuximab | IL-6 | 1–2 | 5.5/11 mg/kg | ≥9 | Siltuximab + Bor (1.3 mg/m2)+ Dex (20 mg) | CR = 22%; PR = 44% | [38] |
| Orlowski et al. (2015) Phase II | 142 | Siltuximab + Bor | IL-6 | 1–3 | Siltuximab 6 mg/kg | 4 | MoAb + Bor | mPFS = 8 m; ORR = 55%; CR = 11%; OS = 30.8 m | [36] |
| 139 | Bor + placebo | Placebo + Bor | mPFS = 7.6; ORR = 47%; CR = 7%; OS = 36.8 m | ||||||
| Voorhees et al. (2009) Phase II | 14 | Siltuximab | IL-6 | 4 | 6 mg/kg | 4 | MoAb monotherapy | No response (CR/PR); SD = 62%; PD = 39% | [37] |
| 39 | Siltuximab + Dex | 6 mg/kg + 40 g | Siltuximab + Dex | ORR = 23%; PR = 17%; MR = 6%; SD = 57%; PD = 17%; PFS = 3.7 m | |||||
| Raje et al. (2017) Phase II | 74 | Tabalumab | BAFF | 1–3 | 100 mg | 8 or 10 | Tab + Bor + Dex | ORR = 58.1% | [41] |
| 74 | Tabalumab | 300 mg | Tab + Bor + Dex | ORR = 59.5% | |||||
| 72 | Placebo | No MoAb | Placebo + Bor + Dex | ORR = 61.6% | |||||
| Iida et al. (2016) Phase I | 16 | Tabalumab | BAFF | 1–5 | 100–300 mg | ≥9 | Tab + Bor (1.3 mg/m2) + Dex (20 mg/day) | ORR = 56.3%; CR = 0%; VGPR = 18.8%; SD = 1.3%; PD = 18.8% | [42] |
| Rossi et al. (2009) Phase I | 12 (11 REP) | Atacicept | BAFF | NR | 2–10 mg/kg | 5 | MoAb monotherapy | No ORR; PD = 54%; SD = 45% | [43] |
| White et al. (2013) Phase II | 49 | Bevacizumab + Bor | VEGF | 1–3 | Bevacizumab 15 mg/kg iv. | 8 | MoAb + Bor | ORR = 51%; PR = 16.3%; mPFS = 6.2 m | [44] |
| 53 | Bor + placebo | Bor 1.3 mg/m2 | Placebo + Bor | ORR = 43.4%; PR = 7.5%; mPFS = 5.1 m | |||||
| Somlo et al. (2011) Phase II | 6 | Bevacizumab | VEGF-A | 3 (0–5) | Bevacizumab 10 mg/kg | 4 | Monotherapy | PD = 29–69 days; SD = 238 days; SD = 16.6%; PD = 83% | [45] |
| 6 | Bevacizumab ± thalidomide | VEGF-A | 4 | Bevacizumab ± thalidomide | SD = 37–350 days; PR = 33%; PD = 67% | ||||
| Badros et al. (2017) Phase II | 48 | Pembrolizumab | PD-1 | 3 (2–5) | 200 mg iv. ×2 wk | 28 | Pem + Pom + Dex | mPFS = 17.4 m; OR = 60%; VGPR = 19%; PR = 33% | [46] |
| Lesokhin et al. (2016) Phase Ib | 27 | Nivolumab | PD-1 | 3 (1–12) | 1–3 mg/kg ×2 wk | NR | MoAb monotherapy | mPFS = 10 wk; OR = 4%; SD = 63%; CR = 4% | [47] |
| Efebera et al. (2015) Phase I/II | 12 | Pidilizumab | PD-1 | 2 (2–11) | 1.5–6 mg/kg every 28 days | NR | Pidilizumab + Len (15–25 mg) | VGPR: n = 3; PR: n = 1 | [48] |
| Berdeja et al. (2012), Phase I | 44 (39 REP) | Lorvotuzumab mertansine | CD56 | 2 (1–11) | 75–112 mg/m2 | NR | LM + Len (20 mg) + Dex (40 mg) | ORR = 59%; sCR: n = 1; CR: n = 1; VGPR: n = 8; PR: n = 9 | [49] |
| Chanan et al. (2010) Phase I | 37 | Lorvotuzumab mertansine | CD56 | 6 | 40–140 mg/m2 ×1 wk | NR | Monotherapy | SD = 41% | [50] |
| Kelly et al. (2016) Phase I/IIa | 47 (43 REP) | Indatuximab ravtansine (ADC) | CD138 | 1–6 | 80–100 mg/m2 | NR | Indatuximab + Dex + Len | ORR = 78%; PR = 33/47; mPFS 16.4 m | [51] |
| 17 | >2 | NR | Indatuximab + Dex + pomalidomide | ORR = 79%; VGPR = 4; PR = 7 | |||||
| Kelly et al. (2014) Phase I/IIa | 45 (36 REP) | Indatuximab ravtansine (ADC) | CD138 | 3 | 80, 100, 120 mg/m2 | NR | Indatuximab + Len + Dex | ORR = 78%; sCR = 1; CR = 2; VGPR = 10; PR = 15; SD = 2 | [52] |
| Heffner et al. (2012) Phase I/IIa | 29 (23 REP) | Indatuximab ravtansine (ADC) | CD138 | 2 (1–11) | 40–160 mg/m2 | NR | Monotherapy | PR = 1; SD = 11; mPFS = 112 days (90–245) | [53] |
ADC: Antibody drug conjugate; BAFF: B-cell activating factor; Bor: Bortezomib; CR: Complete response; Dex: dexamethasone; Len: Lenalidomide; LM: Lorvotuzumab mertansine; m: month; MoAb: Monoclonal antibody; mPFS: Median progression free survival; MR: Minimal response; NE: Not evaluable; NR: Not reported; OR: overall response; ORR: Objective response rate; OS: Overall survival; PD: Progressive disease; Pem: Pembrolizumab; Pom: Pomalidomide; PR: Partial response; REP: Response evaluable patient; sCR: Stringent complete response; SD: Stable disease; Tab: Tabalumab; VEGF: Vascular endothelial growth factor; VGPR: Very good partial response; wk: Week.
BAFF
BAFF and a proliferation inducing ligand (APRIL) are members of the TNF-α family. In MM patients, BAFF, APRIL, TNF-α and their receptors are present in higher concentrations [54] and their levels provide statistically significant correlation with disease activity [55]. These factors activate NF-κ phosphatidylinositol-3 kinase and protect MM cells from Dex-induced apoptosis [54]. Two anti-BAFF MoAb, atacicept and tabalumab have been tested. Atacicept blocks both BAFF (soluble form) and APRIL [43]. Tabalumab blocks BAFF (both membrane bound and soluble forms). Rossi et al. studied atacicept monotherapy in 12 RRMM with no OR reported [43]. For tabalumab, we summarized data from two trials each testing tabalumab in combination with bortezomib and Dex. Shinsuke et al. reported an OR of 57% with tabalumab plus bortezomib and dexamethasone (Vd) in RRMM patients with 1–5 prior lines of therapy. Raje et al. found no significant difference in PFS and OR between tabalumab 100 mg, tabalumab 300 mg and placebo, each tested in combination with Vd. ORRs were 58.1, 59.5 and 61.1% with tabalumab 100 mg plus Vd, tabalumab 300 mg plus Vd and placebo plus Vd, respectively [41]. Patients with low BAFF levels had comparatively longer PFS so BAFF may play a role as a prognostic marker in RRMM patients (Table 2) [41].
Vascular endothelial growth factor
Bevacizumab, a MoAb, blocks angiogenic and proliferative effects of VEGF by binding with soluble VEGF [56]. We summarized data from three trials with bevacizumab in RRMM patients. Callander et al. studied bevacizumab in combination with lenalidomide and Dex in 31 RRMM patients with median 3 prior lines of therapy and reported an OR of 70% [57]. However, the outcome was not superior to lenalidomide and Dex without bevacizumab (OR of 60%) [58]. In a head-to-head comparison of bevacizumab (n = 6) versus bevacizumab ± thalidomide (n = 6) in patients who had median three prior lines of therapy, Somlo et al. reported no OR with bevacizumab monotherapy. A PR of 33% was reported with bevacizumab ± thalidomide [45]. White et al. in a head-to-head comparison of bevacizumab plus bortezomib (n = 49) versus bortezomib plus placebo (n = 53) in RRMM patients who had 1–3 prior lines of therapy, found no statistically significant difference in ORR (51 vs 43.3%) (Table 2) [44].
Immune checkpoint inhibitors
PD-1/PD-L1
PD-1 axis is involved in regulating T-cell activation and their apoptotic pathways. PD-1 is expressed on T-cell surface and its ligand PD-L1 on tumor cells. Their interaction inhibits T-cell proliferation. MoAb against PD-1/PD-L1 disinhibit T-cell proliferation and blocks immune escape by tumor cells [59]. We summarized results from three studies with anti-PD-1 MoAb (nivolumab, pembrolizumab and pidilizumab) in RRMM patients. Lesokhin et al. studied nivolumab in 27 RRMM patients with median three prior lines of therapy. Only one patient achieved OR [47]. Badros et al. studied pembrolizumab in combination with Pom and Dex in 48 RRMM patients with median three prior lines of therapy. Objective response was seen in 29/48 (60%) patients: 4 stringent CR (sCR), 9 VGPR and 16 PR. Median PFS of 17.4 months was seen [46]. Efebera et al. studied pidilizumab and lenalidomide in 12 RRMM patients with median two prior lines of therapy. One patient achieved PR and three achieved VGPR [48]. Kelly et al. showed pretreatment with oncolytic virus reolysin, through IFNγ/JAK/STAT axis, primarily regulated induction of PD-L1, induction of PD-L2 expression and improved anti-PD-L1 therapy in murine model (Table 2) [60].
Antibody–drug conjugate
CD56
CD56 is a cell surface glycoprotein, first in line ADC. It is also called neuronal cell adhesion molecule as it is highly expressed on neuronal cells, NK cells and cytotoxic T cells [61,62]. CD56 expression is absent on benign B cells and can be present on more than 70% myeloma cells [50]. Lorvotuzumab is an anti-CD56 antibody that is conjugated with a maytansinoid cytotoxic agent called DM-1. We summarized results from two studies on lorvotuzumab in RRMM patients. Chanan-Khan et al. studied lorvotuzumab mertinase as monotherapy in 37 heavily pretreated RRMM patients with 6 prior lines of therapy and no OR was reported in their study. Berdeja et al. studied lorvotuzumab mertinase in combination with lenalidomide and Dex in 44 RRMM patients with median 2 prior lines of therapy. An ORR of 58%, 1 sCR, 1 CR and 8 VGPR were noted among 32/44 response evaluable patients (Table 2). The most common adverse effect noted was peripheral neuropathy. The spectrum of its toxicity can be explained by the distribution of CD56 on normal neuronal and NK cells [49].
CD138
CD138 is expressed on malignant cells in hematologic malignancies and solid tumors and is important for cell-to-cell adhesion as it acts as a co-receptor for MM growth factors [63]. In heavily pretreated patients, it is an important and reliable marker. Indatuximab ravtansine (IR, BT-062) is an immune conjugate of anti-CD138 chimerized MoAb and cytotoxic maytansinoid DM4. When BT062 binds to CD138, it is internalized and releases cytotoxic DM4, an antimicrotubule agent leading to death of MM cells. We summarized results from three studies on this ADC in RRMM patients. Heffner et al. studied IR as monotherapy in 29 RRMM patients, one patient achieved OR [53]. Kelly et al. studied this ADC in combination with lenalidomide and Dex in 64 RRMM patients with 3 median prior lines of therapy and reported an OR of 78% [52]. Kelly et al. in a head-to-head comparison of IR + lenalidomide and dexamethasone (Rd) versus IR + Pom and Dex found no significant difference in OR (78 vs 79%) (Table 2) [51].
CAR T-cell targets
Our literature search identified a total of 287 articles on CAR T cells: PubMed: 46, Cochrane: 5, EMBASE: 105, Scopus: 42 and Web of Science: 89. After detailed scrutiny, we included six studies while manual citation analysis retrieved one additional study. A total of 7 studies with 86 RRMM patients were included in our review of CAR T-cells target therapy. Targeted antigens included BCMA (n = 4 studies), CD138 (n = 1), CD19 (n = 1) and kappa light chain (n = 1) (Table 3).
Table 3. . Chimeric antigen receptor T cells in multiple myeloma.
| Author (year) study design | Number of patients | Median number of prior therapy lines | Lympho-depletion therapy | CAR T-cell type | Intracellular domain | CARTs dose | Targeted antigen, location | Outcome | Cytokine-release syndrome | Other adverse effects | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ali et al. (2016) Phase I | 12 | 7 | CF | CAR-BCMA | CD28 ICD-CD3zeta | 0.3–9 × 106/kg | BCMA, normal plasma cells, malignant plasma cells, some B cells | 0.3 × 106 = 1 PR (2 wk), 2 SD (6 wk) 1 × 106 = 3 SD (2–12 wk) 3 × 106 = 1 VGPR (8 wk), 3 SD (2–8 wk) 9 × 106 = 1 sCR (17 wk), 1 VGPR (26+ wk) |
1 × 106 = mild (2 pt) 3 × 106 = mild (3 pt) Severe (1 pt) 9 × 106 = severe (2 pt) (required tocilizumab) |
Anemia, neutropenia, nausea, hypocalcemia, hypophosphatemia | [64] |
| Fan et al. (2017) Phase I | 35 (19 REP) |
Not specified | Not reported | LCAR-B38M | NS | 0.6–7 × 106/kg | As above | ORR: 100% 14 sCR (8 wk) 1 PR (8 wk) 4 VGPR (8 wk) |
Overall 74% 9 Grade I 2 Grade II 1 Grade III 1 Grade IV |
Anemia fever dyspnea | [65] |
| Cohen et al. (2016) Phase I | 6 | 9 | Not reported | CAR-BCMA | 4-1BB ICD-CD3zeta | 1–5 × 108/pt | As above | 1.8 × 108 = 1 PD 2 × 108 = 1 VGPR (5 m) 1 sCR (7+ m) 5 × 108 = 1 MR (1+ m) 1 MR (2 m) 1 SD (2 m) |
83% 2 Grade I 1 Grade II 2 Grade III (required tocilizumab) |
Anemia, neutropenia, pleural effusion (16%), hypocalcemia (33%) hypophosphatemia (50%) PRES: 1 Grade IV |
[66] |
| Berdeja et al. (2016) Phase I | 9 | 6 | CF | bb2121 anti-BCMA CAR T cells | 4-1BB ICD-CD3zeta | 5–45 × 107 cells | As above | 5 × 107 = 1 PR (2.9 m) 1 SD (2.1 m) 1 PD (2 m) 15 × 107 = 1 sCR (6.4 m) 1 sCR (4.8 m) 1 VGPR (4.4 m) 45 × 107 = 1 PR (3 m) 1 SD (2.5 m) 1 PR (1 m) |
67% Grade I/II | Neutropenia (89%), leukopenia (67%), anemia (44%) | [67] |
| Guo et al. (2015) Phase I | 5 | 5–8 | PCD, CP, VAD | CART-138 | CD28 ICD-CD3zeta | 0.44–1.51 × 107/kg | CD 138 (syndecan 1), neoplastic and Non-neoplastic plasma cells | 4 SD (3–7 m) 1 PD |
Not reported | 4 Grade III fever | [68] |
| Ramos et al. (2016) Phase I | 7 | ≥1 (2–8) | C | Kappa-CAR T cells | CD28 ICD-CD3zeta | 0.2–2 × 108/m2 | Kappa light chain, mature B cells | 4 SD 3 Not reported |
None | Grade III lymphopenia | [69] |
| Garfall et al. (2016) Pilot study | 12 | 6 | Not reported | CTL019 cells | 4-1BB ICD-CD3zeta | 1–5 × 107 cells Additional therapy: ASCT + Melphalan |
CD-19. Rarely on MM Plasma cells and cancer stem cell population | Median PFS = 6 m 1 CR 6 VGPR (3.2 m) 2 PD (3.2 m) 2 PR (3.2 m) |
1 Grade I | Grade 3 GVHD: n = 1; Grade 3 oral mucositis: n = 1 | [70] |
ASCT: Autologous stem cell transplantation; BCMA: B-cell maturation antigen; C: Cyclophosphamide; CART: Chimeric antigen receptor modified T cell; CF: Cyclophosphamide, fludarabine; CP: Carboplatin, paclitaxel; CR: Complete response; GVHD: Graft versus host disease; m: Month; MM: Multiple myeloma; MR: Minimal response; ORR: Overall response rate; PCD: Bortezomib, cyclophosphamide, dexamethasone; PD: Progressive disease; PFS: Progression-free survival; PR: Partial response; PRES: Posterior reversible encephalopathy syndrome; pt: Patient; REP: Response evaluable patient; sCR: Stringent complete response; SD: Stable disease; VAD: Vincristine, doxorubicin, dexamethasone; VGPR: Very good partial response; wk: Week.
B-cell maturation antigen
BCMA receptor, a member of TNF family, helps B cells to differentiate into plasma cells and is highly expressed on malignant plasma cells and some B cells. It is not expressed on hematopoietic stem cells and nonhematopoietic tissues [71]. To date, 62 patients in 4 different trials received BCMA CAR T dose ranging from 3 × 106 to 5 × 108. A maximum ORR of 100% was reported in one study [65]. Among other adverse effects, CRS was a major concern with use of BCMA CAR T cells, and preliminary results are very encouraging about its efficacy.
In a Phase I dose escalation trial, 12 patients with RRMM received second-generation BCMA-CAR T cells with CD28 ICD-CD3 zeta intracellular domain. Among two patients who received maximum dose of 9 × 106 CAR T cells, one had stringent CR (sCR) for 17 weeks and other had an ongoing VGPR. The clinical response was lower in patients treated with lower doses of BCMA-CAR T cells. The main toxicity concern was CRS, and prolonged cytopenia in patients treated with higher doses [64]. In another Phase I trial, six patients received split does of BCMA-CAR T cells. Patients were divided in three cohorts and two cohorts among them also received cyclophosphamide (CTX) as preconditioning or lymphodepletion therapy. One patient had Posterior Reversible Encephalopathy Syndrome treated with anti-epileptics, methylprednisolone and CTX. Totally, 5 patients (83%) had CRS and 2 of them required treatment with IL-6 blockage (tocilizumab). One patient who received 2 × 108 CAR T cells had ongoing sCR for 7 months and one patient achieved VGPR at 5 months. In terms of disease-related challenges, evidence of antigen escape was noted by loss of BCMA expression [66]. Garfall et al. have also reported a case of steroid refractory PRES when the patient received 2 × 108 41BB-CD3 zeta CART BCMA cells. She developed fever and altered mental status and steroids showed no benefit. She received CTX and showed symptomatic improvement [72].
In another Phase I trial, LCAR-B38M CAR T cells were infused in 19 patients with RRMM. Totally, 100% ORR was achieved. A total of 18 out of 19 patients had CR for a 6-month follow up period. A total of 14 out of 19 patients experienced CRS including one case each of Grades III and IV CRS [65]. Bb2121 is a CAR T cell construct with 41BB co-stimulatory motif. In a Phase I trial, nine patients with RRMM were given these CAR T cells after lymphodepletion with CTX and fludarabine. Three patients who were treated with 5 × 107 CAR T cells achieved PR (1) and SD (2). Two out of three patients on 15 × 107 CAR T-cells dose had sCR for 21+ and 18+ months. In addition to CRS in 67% patients, 89% had neutropenia, 67% had leukopenia and 44% had anemia (Table 3) [67].
CD19
CD19 is expressed in a minor subset of drug-resistant MM as well as on precursor myeloma cells. Elimination of CD19 expressing cells can prevent MM recurrence [73]. The use of CD19-targeting CAR T cells in conjunction with standard of care therapy for MM has shown clinical benefit. Garfall et al. reported a patient who previously had MM refractory to melphalan and autologous stem cell transplantation (ASCT). Patient was again treated with melphalan and second ASCT followed by 5 × 107 CTL019 CAR T cells, which were infused 12 days after transplant. Patient maintained CR and no progression was detected for 12 months after treatment [74]. In another Phase I trial, 10 out of 12 patients received up to 5 × 107 CD19-directed CAR T cells after melphalan and ASCT. The therapy was well tolerated with incidence of only one Grade I CRS reported. A mean PFS of 185 days was achieved but all patients eventually progressed. The mean PFS of ASCT with CTL019 therapy exceeded PFS of primary ASCT alone (Table 3) [70]. A Phase II trial testing CTL019 post-ASCT is still ongoing (Clinialtrials.gov ID: NCT02794246).
Kappa light chain
B cells and plasma cells can express either kappa or lambda chains on their surface. In a Phase I study with selective targeting for kappa light chain, CAR T cells (k.CAR-T) with CD28 ICD-CD3 zeta intracellular domain were used in doses ranging from 0.2–2 × 108/m2. Patients showed 50% reduction of B cells 2 weeks after treatment initiation. Out of seven patients who received k.CAR-T cells, four patients showed SD. After infusion, k.CAR-T cells were well tolerated and no CRS was reported [69]. This light chain selective therapy can be useful in the future to maximize antitumor effect without causing B-cell aplasia and further clinical trials are required to study this therapy (Table 3).
CD138
CD138 is highly expressed on plasma cells and CAR T-cell therapy against CD138 was tested by Guo et al. in a Phase I clinical trial. Five patients were treated with CAR T cells with CD28 ICD-CD3 zeta intracellular domain in dose ranging from 0.44–1.5 × 107/kg, preconditioning regimen included multi-agent chemotherapy. Four patients showed SD for 3–7 months. In one patient, CD138 myeloma cells decreased from 10.5% to 1–3% in peripheral blood. A consistent increase of CD138 CAR T cells was noted in bone marrow after 7 weeks of initial treatment (Table 3) [68].
Role of antibodies in newly diagnosed MM and side effect profiling is summarized in Tables 4 & 5, respectively.
Table 4. . Monoclonal antibodies in newly diagnosed multiple myeloma.
| Author (year) study design | Number of patients | Antibody | Target | Dose | Number of cycles | Regimen | Outcome | Ref. |
|---|---|---|---|---|---|---|---|---|
| San-Miguel et al. (2014) Phase II | 54 | Placebo | IL-6 | 11 mg/kg siltuximab | 9 | VMP | ORR = 80%, CR = 22%, PR = 57%, VGPR = 51%, MR = 8%, PD = 0% | [39] |
| 52 | Siltuximab | Siltuximab + VMP | ORR = 88%, CR = 27%, PR = 61%, VGPR = 71%, MR = 2%, PD = 0% | |||||
| Shah et al. (2016) Phase I/II | 11 (9 REP) | Siltuximab | IL-6 | 8.3–11 mg/kg | 4 | Siltuximab + RVD | ORR = 90.9%, CR = 9.1%, VGPR = 45.5%, PR = 36.45 | [40] |
| Baz et al. (2007) Phase II | 45 | Rituximab + MP | CD20 | 375 mg/m2 | 3 | Rituximab + MP | CR = 0, PR = 58%, MR = 13%, SD = 18%, PD = 11% | [75] |
CR: Complete response; MR: Minimal response; MP: Melphalan, prednisone; ORR: Objective response rate; PD: Progressive disease; PR: Partial response; REP: Response evaluable patient; RVD: Lenalidomide, bortezomib, dexamethasone; VGPR: Very good partial response; VMP: Bortezomib, melphalan and prednisone.
Table 5. . Adverse effects of antibody drugs.
| Author (year) study design | Antibody | Adverse effects ≥ Grade III | Common adverse effects | Ref. |
|---|---|---|---|---|
| Hansson et al. (2015) Phase I | BI-505 | Headache (n = 4), pyrexia (n = 3), infusion-related reactions (n = 1), fluid overload (n = 1), T-wave inversion (n = 1) | Fatigue (47%), pyrexia (32%), headache (32%), nausea (29%), chills (24%) | [24] |
| Callander et al. (2009) Abstract | Bevacizumab | DVT (n = 3), SOB (n = 2), atrial fibrillation (n = 3) | Fatigue | [57] |
| Somlo et al. (2011) Phase II | Bevacizumab | Fatigue (16.6%), HTN (16.6%), neutropenia (16.6%), hyponatremia (16.6%) | Not reported | [45] |
| Bevacizumab ± thalidomide | Lymphopenia (16.6%), fatigue (16.6%), pulmonary HTN (16.6%) | |||
| White et al. (2013) Phase II | Bevacizumab + bortezomib | Thrombocytopenia (28%), neutropenia (18%) | Anemia, diarrhea, fatigue, URTI, neuralgia | [44] |
| Bortezomib + placebo | Thrombocytopenia (30%), diarrhea (10%) | Anemia, diarrhea, fatigue, URTI, neuralgia | ||
| Rasche (2015) Phase I | PAT-SM6 | Neutropenia (8.3%), back pain (8.3%), bile duct stone (8.3%) | Neutropenia (50), leukopenia (67) | [76] |
| Orlowski et al. (2015) Phase II | Siltuximab + bortezomib | Neutropenia (49%), thrombocytopenia (48%) | Infections (62%), sensory neuropathy (49%) | [36] |
| Bortezomib + placebo | Neutropenia (24%), thrombocytopenia (34%) | Infections (49%), sensory neuropathy (51%) | ||
| Agura et al. (2009) Phase Ib | Dacetuzumab | Herpes zoster, renal failure | Infusion reactions, Grade I/II, fatigue (47%), neutropenia (28%), thrombocytopenia (25%), diarrhea (22%), constipation (19%), headache (19%) | [21] |
| Hussein et al. (2010) Phase I | Dacetuzumab | Total Grade III AE = 30%, thrombocytopenia (7%), aseptic meningitis (5%), renal failure (5%) | Fatigue (57%), headache (43%), nausea (23%), anemia (21%). Elevated LFTs (41%), anorexia, back pain, constipation, diarrhea, ocular hyperemia (21%) | [22] |
| Bensinger et al. (2012) Phase I | Lucatumumab | Thrombocytopenia (4%), increased LFTs (4%), increased lipase (4%) | Infusion reactions, anemia (7%), hypercalcemia (7%), pyrexia (7%) | [23] |
| Martin et al. (2014) Phase I | Isatuximab (SAR650984) | Pneumonia, fever, hyperglycemia, hypophosphatemia | Pneumonia (9%), fever (3%), apnea (3%), fatigue (3%), hyperglycemia (3%) | [11] |
| Martin et al. (2014) Phase Ib | Isatuximab SAR650984 | No DLT reported, IAR (6%) | Fatigue (41.9%), nausea (38.7%), URTI (38.7%), diarrhea (35.5%) | [13] |
| Richter et al. (2016) Phase II | Isatuximab SAR650984 | Not reported | Nausea (33%), fatigue (30%), dyspnea (26%), infusion related (49%) | [12] |
| Voorhees et al. (2009) Phase II | Siltuximab | Thrombocytopenia, anemia, neutropenia, abnormal LFTs, fatigue | Diarrhea (29%), nausea (22%), constipation (20%), fatigue (43%), peripheral edema (29%) | [37] |
| Siltuximab + dexamethasone | ||||
| Suzuki et al. (2015) Phase I | Siltuximab | No DLT, lymphopenia (89%), neutropenia (44%) | Abnormal LFTs (44%), rash (44%), hyperlipidemia (44%) | [38] |
| Rossi et al. (2009) Phase I | Atacicept | Neuropathy, epiploic appendicitis. | Infections, bone pains | [43] |
| Iida et al. (2016) Phase I | Tabalumab | Febrile neutropenia, tumor lysis syndrome, Ileus | Thrombocytopenia (81.3%), lymphopenia (43.8%), increased alanine aminotransferase (43.8%) | [77] |
| Raje et al. (2017) Phase II | Tabalumab | Thrombocytopenia (12 8%), pneumonia (9.1%) | Thrombocytopenia (37%), fatigue (37%), diarrhea (35%), constipation (32%) | [41] |
| Placebo | ||||
| Lesokhin et al. (2016) Phase Ib | Nivolumab | Pneumonitis (4%), myositis (4%), raised CPK (4%) | Seen in 52% patients | [47] |
| Badros et al. (2017) Phase II | Pembrolizumab | Hematologic (40%), hyperglycemia (25%), pneumonia (15%) | Pancytopenia (13%), hypothyroidism (10%) | [46] |
| Efebera et al. (2015) Phase I/II | Pidilizumab | Anemia 25%, neutropenia 23%, thrombocytopenia 34% | Fatigue (50%), anorexia (17%), hypophosphatemia (17%) | [48] |
| Chanan-Khan et al. (2010) Phase I | Lorvotuzumab mertansine | Peripheral neuropathy, fatigue, acute renal failure | Fatigue, peripheral neuropathy, headache, raised AST | [50] |
| Berdeja (2012) Phase I | Lorvotuzumab mertansine | Peripheral neuropathy, neutropenia (n = 1), hyperuricemia tumor lysis syndrome (n = 2) | Peripheral neuropathy (42%) | [49] |
| Heffner et al. (2012) Phase I/IIa | Indatuximab ravtansine (ADC) | Palmar-planter erythrodysesthesia syndrome (n = 1), elevated LFTs | Fatigue, anemia, diarrhea | [53] |
| Kelly et al. (2014) Phase I/IIa | Indatuximab ravtansine (ADC) | Mucosal inflammation (n = 1), anemia (n = 1) | Fatigue, hypokalemia, diarrhea | [52] |
| Kelly et al. (2016) Phase I/IIa | Indatuximab ravtansine (ADC) | Not reported | Diarrhea, fatigue, nausea | [51] |
| Benson et al. (2015) Phase I | IPH 2101 | Leucopenia (n = 1), neutropenia (n = 1) | Myelodysplasia (n = 1), neutropenia, IRR | [20] |
| Benson et al. (2012) Phase I | IPH 2101 | Not reported | Fatigue (n = 10), chills (n = 5), pyrexia (n = 5) | [19] |
| Kaufman et al. (2013) Phase I | Milatuzumab | Anemia 20%, CRS 4%, hypokalemia 4%, epistaxis 4% | Nausea (48%), fever (36%), CRS (20%), headache (20%), HTN (20%) | [16] |
| Lacy et al. (2008) Phase I | Figitumumab (CP 751, 871) | Anemia (2.1%), hyperglycemia (2.1%) | Anemia (6.4%), increased AST (6.4%) | [18] |
| Moreau et al. (2011) Phase I | AVE1642 | Hypercalcemia (n = 1), renal vein thrombosis (n = 1) | Not reported | [17] |
| San-Miguel et al. (2014) Phase II | VMP + placebo | All = 81%, neutropenia (43%), thrombocytopenia (25%), pneumonia (17%), median PFS = 17 months | Infections (17%), GI disorders (11%) | [39] |
| Siltuximab + VMP | All = 92%, neutropenia (62%), thrombocytopenia (44%), pneumonia (17%), median PFS = 17 months | Infections (29%), GI disorders (11.5%) | ||
| Shah et al. (2016) Phase I/II | Siltuximab | Pneumonia, thrombocytopenia | Fatigue (63.6%), constipation (54.5%), paresthesia (45.5%), myalgia (56.4%) | [40] |
| Baz et al. (2007) Phase II | Rituximab + MP | Diarrhea (31%), neutropenia (51%), anemia (47%), thrombocytopenia (40%) | Fever, fatigue, cough, dyspnea, diarrhea, nausea, diarrhea and constipation. Possible AE related to rituximab were IRR (11%) | [75] |
ADC: Antibody drug conjugate; AE: Adverse event; CRS: Cytokine release syndrome; DLT: Dose-limiting toxicity; DVT: Deep venous thrombosis; GI: Gastrointestinal; HTN: Hypertension; IAR: Infusion-associated reaction; IRR: Infusion-related reaction; LFT: Liver function test; m: Month; MP: Melphalan, prednisone; PFS: Progression-free survival; SOB: Shortness of breath; URTI: Upper respiratory tract infection; VMP: Bortezomib, melphalan and prednisone.
Future perspective
The use of IMiDs and PIs has improved OS in MM but most patients will face relapse and refractory disease. Immune therapy is a relatively new but promising treatment modality for MM. Elotuzumab and daratumumab are two antibodies which are FDA approved for the treatment of MM. In our review, ISA (anti-CD38 antibody) was the only MoAb with encouraging (ORR: 24%) results as monotherapy. Most other MoAb therapies did not show promising results as monotherapy. CD38 represents a promising immunotherapy target for future trials and ISA data in combination trials is very promising for MM. As elotuzumab had limited efficacy as monotherapy, but showed efficacy in combination with other agents (ELOQUENT – 2 trial) [78], other MoAbs that failed to produce any OR as monotherapy should be tested further in combination therapy.
The MMY3004 (CASTOR) trial (bortezomib and Dex ± daratumumab) [79] and MMY3003 (POLLUX) trial (lenalidomide and Dex ± daratumumab) [80] revealed that addition of daratumumab led to superior outcomes for MM. Considering the immunomodulatory properties of IMiDs and superior efficacy with PI combination therapy, it seems reasonable to combine these agents with novel MoAb and evaluate their efficacy. Combination therapy using siltuximab, indatuximab, ISA, pembrolizumab, dacetuzumab and lorvotuzumab with IMiDs and PIs have produced positive results.
ADC seems an appealing treatment modality because of targeted nature of therapy. Indatuximab did not show activity as a single agent but produced encouraging results as ADC (IR), which highlights the need to test other MoAb as ADC.
CAR T-cell therapy has shown very encouraging results with BCMA CAR T cells being the one with most clinical efficacy. However, the CAR T toxicity profile remains a major concern. CD19 CAR T cells recently received FDA approval for ALL. Research data and lessons from drug development for ALL can pave the way for CAR T cells in the field of lymphomas, chronic leukemias, MM and other malignancies with a potential immune targets.
We conclude that with the improvement in the understanding of biological drivers, pathogenesis, role of tumor microenvironment, immunological targets, role of checkpoint inhibitors and immunotherapy for MM using MoAbs, CAR T cells will continue to evolve either as single agent, combination therapy or as sequential therapy. In the near future, with the aim to achieve cure or functional cure for MM, immunotherapy will play a bigger role for induction, consolidation and maintenance therapy.
Executive summary.
Monoclonal antibodies (MoAbs) exert their antitumor effects through several mechanisms including antibody-dependent cellular cytotoxicity, complement-dependent cytotoxicity, antibody drug conjugate and recruitment of immune cells like NK cells.
Daratumumab and elotuzumab have been US FDA approved for treatment of relapsed refractory multiple myelom (RRMM). Randomized Phase III trials (CASTOR/POLLUX/ELOQUENT-2) show higher efficacy in combination setting with immunomodulatory drugs and proteasome inhibitors (PIs). A large number of additional MoAbs are under evaluation either as single agent or as combination modality in early Phase clinical trials.
Isatuximab is the only MoAb with encouraging (objective response rate: 24%) results as monotherapy. Most of MoAbs included in our review, such as milatuzumab (anti-CD74), AVE1642 and figitumumab (anti-IGF-1R), IPH 2101 (antikiller-cell immunoglobulin like receptor), dacetuzumab and lucatumumab (anti-CD40), PAT-SM6 (anti-GRP-78), BI 505 (anti-intercellular adhesion molecule-1), siltuximab (anti-IL-6), atacicept (anti-B-cell activating factor [anti-BAFF]), bevacizumab (antivascular endothelial growth factor) and lorvotuzumab mertansine (anti-CD56), failed to produce promising results when used as monotherapy. Further trials need to test these MoAbs in combination therapy.
MoAbs producing positive outcomes in combination therapy include siltuximab (Siltuximab + bortezomib and dexamethasone; overall response [OR]: 66%), indatuximab (Indatuximab + lenalidomide and dexamethasone [Rd]; OR: 78%), isatuximab (Isatuximab + Rd; OR: 64.5%), pembrolizumab (Pembrolizumab + Pd; OR: 60%), bevacizumab (Bevacizumab + Rd; OR: 70%), dacetuzumab (Dacetuzumab + Rd; OR: 39%) and lorvotuzumab (Lorvotuzumab + Rd; OR: 56.4%).
MoAb-based combination regimens whose outcomes were not statistically significant include bevacizumab + Rd, bevacizumab + bortezomib, siltuximab + bortezomib and siltuximab + bortezomib, melphalan and prednisone. Role of anti-PD1 MoAb and indatuximab (anti-CD138) as monotherapy is questionable and needs further evaluation.
Preclinical studies of TTI-621(SIRPαFc), an anti-CD47 antibody, in combination with PIs showed greater antimyeloma activity in combination as compared with monotherapy. These data provide a rational to test this antibody in human patients.
Atacicept and siltuximab were associated with increased incidence of infections. Higher incidence of neuropathy was reported with antibody drug conjugate (lorvotuzumab mertansine), which is concerning as most of RRMM patients have pre-existing PNP (peripheral neuropathy) partially due to disease itself and partially contributed to anti-MM agents (notably bortezomib and thalidomide). Thus, lorvotuzumab mertinase might not be an appealing immune therapy approach for treatment of MM.
Although no MoAb has effectively targeted BAFF, but BAFF can be used as a prognostic tool in RRMM patients.
Oncolytic reovirus is an emerging idea in immunotherapy and has shown to increase the levels of PD-L1 in malignant myeloma cells. It can also sensitize cancer cells with low PD-L1 expression to PD-1/PD-L1 antibody (nivolumab, pembrolizumab, pidilizumab) therapy.
Chimeric antigen receptor (CAR) T-cells targeting B-cell maturation antigen (BCMA), CD19, KLC and CD138 have showed promising results in treatment of heavily pretreated and RRMM patients. CAR T cells acting on BCMA have been proved clinically more efficacious by Fan et al. with objective response rate of 100% (n = 19) but this efficacy is also associated with more severe cytokine release syndrome events. Most patients who received CAR T cells were heavily pretreated with ≥6 median prior lines of therapy. CAR BCMA T cells have also been associated with PRES and neurological deficits which are reversed with cyclophosphamide, steroids and anti-epileptics.
The future of immune therapies in MM is promising, more clinical trials are needed to test MoAb in combination with immunomodulatory drugs, PIs and other MM therapies.
Footnotes
Conflict of interest
Authors declare that there is no conflict of interest with this manuscript.
Financial & competing interests disclosure
This work supported in part by grant P30 CA023074 from the National Cancer Institute, National Institutes of Health, Bethesda, MD, USA. The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript.
Authorship statement
A Sohail, A Mushtaq, A Iftikhar and F Anwer designed the study. All authors performed the study, contributed to data extraction, analyzed the data, wrote the paper and approved the final manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.NIH NCI. Cancer stat facts: myeloma. https://seer.cancer.gov/statfacts/html/mulmy.html
- 2.Society AC. Survival rates by stage for multiple myeloma. 2016. www.cancer.org/cancer/multiple-myeloma/detection-diagnosis-staging/survival-rates.html
- 3.Kumar SK, Lee JH, Lahuerta JJ, et al. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter international myeloma working group study. Leukemia. 2012;26(1):149–157. doi: 10.1038/leu.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hideshima T, Mitsiades C, Tonon G, Richardson PG, Anderson KC. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat. Rev. Cancer. 2007;7(8):585–598. doi: 10.1038/nrc2189. [DOI] [PubMed] [Google Scholar]
- 5.Magarotto V, Salvini M, Bonello F, Bringhen S, Palumbo A. Strategy for the treatment of multiple myeloma utilizing monoclonal antibodies: a new era begins. Leuk. Lymphoma. 2016;57(3):537–556. doi: 10.3109/10428194.2015.1102245. [DOI] [PubMed] [Google Scholar]; •• Elaborates role of elotuzumab and daratumumab and other antibodies, which are in preclinical or early clinical phases of evaluation for treatment of multiple myeloma (MM).
- 6.Ormhoj M, Bedoya F, Frigault MJ, Maus MV. CARs in the lead against multiple myeloma. Curr. Hematol. Malig. Rep. 2017;12(2):119–125. doi: 10.1007/s11899-017-0373-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Reported results of clinical trials conducted utilizing chimeric antigen receptor T-cell therapy in MM.
- 7.Tai YT. Antibody-based therapies in multiple myeloma. In: Munshi N, Anderson K, editors. Advances in Biology and Therapy of Multiple Myeloma. Springer; NY, USA: 2013. [Google Scholar]; • Contains information regarding the role of monoclonal antibodies in MM.
- 8.Scott AM, Allison JP, Wolchok JD. Monoclonal antibodies in cancer therapy. Cancer Immun. 2012;12:14. [PMC free article] [PubMed] [Google Scholar]
- 9.Hoyos V, Savoldo B, Quintarelli C, et al. Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia. 2010;24(6):1160–1170. doi: 10.1038/leu.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fedorov VD, Themeli M, Sadelain M. PD-1- and CTLA-4-based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Sci. Transl. Med. 2013;5(215):215ra172. doi: 10.1126/scitranslmed.3006597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin TG, Hsu K, Strickland SA, Glenn MJ, Mikhael J, Charpentier E. A Phase I trial of SAR650984, a CD38 monoclonal antibody, in relapsed or refractory multiple myeloma. J. Clin. Oncol. 2014;32(Suppl. 15):8532–8532. [Google Scholar]
- 12.Vij R, Martin T, Richter J, et al. Updated data from a Phase II dose finding trial of single agent isatuximab (SAR650984, anti-CD38 MAB) in relapsed/refractory multiple myeloma (RRMM) Haematologica. 2016;101:82–83. [Google Scholar]
- 13.Martin TG, Baz R, Benson DM, et al. A PHASE Ib dose escalation trial of SAR650984 (anti-CD-38 mAb) in combination with lenalidomide and dexamethasone in relapsed/refractory multiple myeloma. Blood. 2014;124(21):83–83. [Google Scholar]
- 14.Richardson PG, Attal M, Miguel JS, et al. A Phase III, randomized, open-label study of isatuximab (SAR650984) plus pomalidomide (Pom) and dexamethasone (Dex) versus Pom and Dex in relapsed/refractory multiple myeloma
- 15.Richardson PG, Mikhael J, Usmani SZ, et al. American Society of Hematology 58th Annual Meeting & Exposition. CA, USA: 3–6 December 2016. Preliminary results from a Phase Ib study of isatuximab in combination with pomalidomide and dexamethasone in relapsed and refractory multiple myeloma. Presented at. [Google Scholar]
- 16.Kaufman JL, Niesvizky R, Stadtmauer EA, et al. Phase I, multicentre, dose-escalation trial of monotherapy with milatuzumab (humanized anti-CD74 monoclonal antibody) in relapsed or refractory multiple myeloma. Br. J. Haematol. 2013;163(4):478–486. doi: 10.1111/bjh.12565. [DOI] [PubMed] [Google Scholar]
- 17.Moreau P, Cavallo F, Leleu X, et al. Phase I study of the anti insulin-like growth factor 1 receptor (IGF-1R) monoclonal antibody, AVE1642, as single agent and in combination with bortezomib in patients with relapsed multiple myeloma. Leukemia. 2011;25(5):872–874. doi: 10.1038/leu.2011.4. [DOI] [PubMed] [Google Scholar]
- 18.Lacy MQ, Alsina M, Fonseca R, et al. Phase I, pharmacokinetic and pharmacodynamic study of the anti-insulinlike growth factor type 1 receptor monoclonal antibody CP-751,871 in patients with multiple myeloma. J. Clin. Oncol. 2008;26(19):3196–3203. doi: 10.1200/JCO.2007.15.9319. [DOI] [PubMed] [Google Scholar]
- 19.Benson DM, Jr, Cohen AD, Jagannath S, et al. A Phase I trial of the anti-KIR antibody IPH2101 and lenalidomide in patients with relapsed/refractory multiple myeloma. Clin. Cancer Res. 2015;21(18):4055–4061. doi: 10.1158/1078-0432.CCR-15-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benson DM, Jr, Hofmeister CC, Padmanabhan S, et al. A Phase I trial of the anti-KIR antibody IPH2101 in patients with relapsed/refractory multiple myeloma. Blood. 2012;120(22):4324–4333. doi: 10.1182/blood-2012-06-438028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agura E, Niesvizky R, Matous J, et al. Dacetuzumab (SGN-40), lenalidomide, and weekly dexamethasone in relapsed or refractory multiple myeloma: multiple responses observed in a Phase Ib study. Blood. 2009;114(22):2870–2870. [Google Scholar]
- 22.Hussein M, Berenson JR, Niesvizky R, et al. A Phase I multidose study of dacetuzumab (SGN-40; humanized anti-CD40 monoclonal antibody) in patients with multiple myeloma. Haematologica. 2010;95(5):845–848. doi: 10.3324/haematol.2009.008003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bensinger W, Maziarz RT, Jagannath S, et al. A Phase I study of lucatumumab, a fully human anti-CD40 antagonist monoclonal antibody administered intravenously to patients with relapsed or refractory multiple myeloma. Br. J. Haematol. 2012;159(1):58–66. doi: 10.1111/j.1365-2141.2012.09251.x. [DOI] [PubMed] [Google Scholar]
- 24.Hansson M, Gimsing P, Badros A, et al. A Phase I dose-escalation study of antibody BI-505 in relapsed/refractory multiple myeloma. Clinical Cancer Res. 2015;21(12):2730–2736. doi: 10.1158/1078-0432.CCR-14-3090. [DOI] [PubMed] [Google Scholar]
- 25.Burton JD, Ely S, Reddy PK, et al. CD74 is expressed by multiple myeloma and is a promising target for therapy. Clin. Cancer Res. 2004;10(19):6606–6611. doi: 10.1158/1078-0432.CCR-04-0182. [DOI] [PubMed] [Google Scholar]
- 26.Bieghs L, Johnsen HE, Maes K, et al. The insulin-like growth factor system in multiple myeloma: diagnostic and therapeutic potential. Oncotarget. 2016;7(30):48732–48752. doi: 10.18632/oncotarget.8982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carlsten M, Korde N, Kotecha R, et al. Checkpoint inhibition of KIR2D with the monoclonal antibody IPH2101 induces contraction and hyporesponsiveness of NK cells in patients with myeloma. Clin. Cancer Res. 2016;22(21):5211–5222. doi: 10.1158/1078-0432.CCR-16-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korde N, Carlsten M, Lee MJ, et al. A Phase II trial of pan-KIR2D blockade with IPH2101 in smoldering multiple myeloma. Haematologica. 2014;99(6):e81–e83. doi: 10.3324/haematol.2013.103085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tong AW, Stone MJ. Prospects for CD40-directed experimental therapy of human cancer. Cancer Gene Ther. 2003;10(1):1–13. doi: 10.1038/sj.cgt.7700527. [DOI] [PubMed] [Google Scholar]
- 30.Schmidmaier R, Mörsdorf K, Baumann P, Emmerich B, Meinhardt G. Evidence for cell adhesion-mediated drug resistance of multiple myeloma cells in vivo . Int. J. Biol. Markers. 2006;21(4):218–222. doi: 10.1177/172460080602100404. [DOI] [PubMed] [Google Scholar]
- 31.Veitonmaki N, Hansson M, Zhan FH, et al. A human ICAM-1 antibody isolated by a function-first approach has potent macrophage-dependent antimyeloma activity in vivo . Cancer Cell. 2013;23(4):502–515. doi: 10.1016/j.ccr.2013.02.026. [DOI] [PubMed] [Google Scholar]
- 32.Wichert S, Juliusson G, Johansson A, et al. A single-arm, open-label, Phase II clinical trial evaluating disease response following treatment with BI-505, a human anti-intercellular adhesion molecule-1 monoclonal antibody, in patients with smoldering multiple myeloma. PLoS ONE. 2017;12(2) doi: 10.1371/journal.pone.0171205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Rhee F, Fayad L, Voorhees P, et al. Siltuximab, a novel anti-interleukin-6 monoclonal antibody, for Castleman's disease. J. Clin. Oncol. 2010;28(23):3701–3708. doi: 10.1200/JCO.2009.27.2377. [DOI] [PubMed] [Google Scholar]
- 34.Voorhees PM, Chen Q, Kuhn DJ, et al. Inhibition of interleukin-6 signaling with CNTO 328 enhances the activity of bortezomib in preclinical models of multiple myeloma. Clin. Cancer Res. 2007;13(21):6469–6478. doi: 10.1158/1078-0432.CCR-07-1293. [DOI] [PubMed] [Google Scholar]
- 35.Voorhees PM, Chen Q, Small GW, et al. Targeted inhibition of interleukin-6 with CNTO 328 sensitizes pre-clinical models of multiple myeloma to dexamethasone-mediated cell death. Br. J. Haematol. 2009;145(4):481–490. doi: 10.1111/j.1365-2141.2009.07647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orlowski RZ, Gercheva L, Williams C, et al. A Phase II, randomized, double-blind, placebo-controlled study of siltuximab (anti-IL-6 mAb) and bortezomib versus bortezomib alone in patients with relapsed or refractory multiple myeloma. Am. J. Hematol. 2015;90(1):42–49. doi: 10.1002/ajh.23868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Voorhees PM, Manges RF, Sonneveld P, et al. A Phase II multicentre study of siltuximab, an anti-interleukin-6 monoclonal antibody, in patients with relapsed or refractory multiple myeloma. Br. J. Haematol. 2013;1161(3):357–366. doi: 10.1111/bjh.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suzuki K, Ogura M, Abe Y, et al. Phase I study in Japan of siltuximab, an anti-IL-6 monoclonal antibody, in relapsed/refractory multiple myeloma. Int. J. Hematol. Res. 2015;101(3):286–294. doi: 10.1007/s12185-015-1743-y. [DOI] [PubMed] [Google Scholar]
- 39.San-Miguel J, Blade J, Shpilberg O, et al. Phase II randomized study of bortezomib-melphalan-prednisone with or without siltuximab (anti-IL-6) in multiple myeloma. Blood. 2014;123(26):4136–4142. doi: 10.1182/blood-2013-12-546374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shah JJ, Feng L, Thomas SK, et al. Siltuximab (CNTO 328) with lenalidomide, bortezomib and dexamethasone in newly-diagnosed, previously untreated multiple myeloma: an open-label Phase I trial. Blood Cancer J. 2016;6:e396. doi: 10.1038/bcj.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raje NS, Moreau P, Terpos E, et al. Phase II study of tabalumab, a human anti-B-cell activating factor antibody, with bortezomib and dexamethasone in patients with previously treated multiple myeloma. Br. J. Haematol. 2017;176(5):783–795. doi: 10.1111/bjh.14483. [DOI] [PubMed] [Google Scholar]
- 42.Iida S, Ogiya D, Abe Y, et al. Dose-escalation study of tabalumab with bortezomib and dexamethasone in Japanese patients with multiple myeloma. Cancer Sci. 2016;107(9):1281–1289. doi: 10.1111/cas.13000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rossi JF, Moreaux J, Hose D, et al. Atacicept in relapsed/refractory multiple myeloma or active Waldenström's macroglobulinemia: a Phase I study. Br. J. Cancer. 2009;101(7):1051–1058. doi: 10.1038/sj.bjc.6605241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.White D, Kassim A, Bhaskar B, Yi J, Wamstad K, Paton VE. Results from AMBER, a randomized Phase II study of bevacizumab and bortezomib versus bortezomib in relapsed or refractory multiple myeloma. Cancer. 2013;119(2):339–347. doi: 10.1002/cncr.27745. [DOI] [PubMed] [Google Scholar]
- 45.Somlo G, Lashkari A, Bellamy W, et al. Phase II randomized trial of bevacizumab versus bevacizumab and thalidomide for relapsed/refractory multiple myeloma: a California Cancer Consortium trial. Br. J. Haematol. 2011;154(4):533–535. doi: 10.1111/j.1365-2141.2011.08623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Badros A, Hyjek E, Ma N, et al. Pembrolizumab, pomalidomide and low dose dexamethasone for relapsed/refractory multiple myeloma. Blood. 2017 doi: 10.1182/blood-2017-03-775122. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 47.Lesokhin AM, Ansell SM, Armand P, et al. Nivolumab in patients with relapsed or refractory hematologic malignancy: preliminary results of a Phase Ib study. J. Clin. Oncol. 2016;34(23):2698–2704. doi: 10.1200/JCO.2015.65.9789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Efebera YA, Rosko AE, Hofmeister C, et al. First interim results of a Phase I/II study of lenalidomide in combination with Anti-PD-1 monoclonal antibody MDV9300 (CT-011) in patients with relapsed/refractory multiple myeloma. Blood. 2015;126(23):1838–1838. [Google Scholar]; • We learn the role of checkpoint inhibitors in treatment of MM.
- 49.Berdeja JG, Ailawadhi S, Weitman SD, et al. Phase I study of lorvotuzumab mertansine (LM, IMGN901) in combination with lenalidomide (Len) and dexamethasone (Dex) in patients with CD56-positive relapsed or relapsed/refractory multiple myeloma (MM) J. Clin. Oncol. 2011;29(15):8013. [Google Scholar]; • Provides facts about the role of antibody drug conjugate in MM.
- 50.Chanan-Khan A, Wolf JL, Garcia J, et al. Efficacy analysis from Phase I study of lorvotuzumab mertansine (IMGN901), used as monotherapy, in patients with heavily pre-treated CD56-positive multiple myeloma – a preliminary efficacy analysis. Blood. 2010;116(21):1962. [Google Scholar]
- 51.Kelly KR, Siegel DS, Chanan-Khan AA, et al. Indatuximab ravtansine (BT062) in combination with low-dose dexamethasone and lenalidomide or pomalidomide: clinical activity in patients with relapsed/refractory multiple myeloma. Blood. 2016;128(22):4486. [Google Scholar]
- 52.Kelly KR, Chanan-Khan A, Heffner LT, et al. Indatuximab ravtansine (BT062) in combination with lenalidomide and low-dose dexamethasone in patients with relapsed and/or refractory multiple myeloma: clinical activity in patients already exposed to lenalidomide and bortezomib. Blood. 2014;124(21):4736. [Google Scholar]
- 53.Heffner LT, Jagannath S, Zimmerman TM, et al. BT062, an antibody-drug conjugate directed against CD138, given weekly for 3 weeks in each 4 week cycle: safety and further evidence of clinical activity. Blood. 2012;120(21):4042–4042. [Google Scholar]
- 54.Moreaux J, Legouffe E, Jourdan E, et al. BAFF and APRIL protect myeloma cells from apoptosis induced by interleukin 6 deprivation and dexamethasone. Blood. 2004;103(8):3148–3157. doi: 10.1182/blood-2003-06-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bolkun L, Lemancewicz D, Jablonska E, et al. BAFF and APRIL as TNF superfamily molecules and angiogenesis parallel progression of human multiple myeloma. Ann. Hematol. 2014;93(4):635–644. doi: 10.1007/s00277-013-1924-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ria R, Vacca A, Russo F, et al. A VEGF-dependent autocrine loop mediates proliferation and capillarogenesis in bone marrow endothelial cells of patients with multiple myeloma. Thromb. Haemost. 2004;92(6):1438–1445. doi: 10.1160/TH04-06-0334. [DOI] [PubMed] [Google Scholar]
- 57.Callander NS, Markovina S, Juckett MB, et al. The addition of bevacizumab (B) to lenalidomide and low dose dexamethasone does not significantly increase response in relapsed or refractory multiple myeloma (NCI#7317) Blood. 2009;114(22):3885–3885. [Google Scholar]
- 58.Weber DM, Chen C, Niesvizky R, et al. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N. Engl. J. Med. 2007;357(21):2133–2142. doi: 10.1056/NEJMoa070596. [DOI] [PubMed] [Google Scholar]
- 59.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kelly K, Espitia C, Zhao W, et al. Oncolytic reovirus sensitizes multiple myeloma cells to anti-PD-L1 therapy. Leukemia. 2017;32:230–233. doi: 10.1038/leu.2017.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goridis C, Brunet J-F. NCAM: structural diversity, function and regulation of expression. Semin. Cell Biol. 1992;3(3):189–197. doi: 10.1016/s1043-4682(10)80015-7. [DOI] [PubMed] [Google Scholar]
- 62.Al-Hujaily EM, Oldham RAA, Hari P, Medin JA. Development of novel immunotherapies for multiple myeloma. Int. J. Mol. Sci. 2016;17(9):26. doi: 10.3390/ijms17091506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dhodapkar MV, Abe E, Theus A, et al. Syndecan-1 is a multifunctional regulator of myeloma pathobiology: control of tumor cell survival, growth, and bone cell differentiation. Blood. 1998;91(8):2679–2688. [PubMed] [Google Scholar]
- 64.Ali SA, Shi V, Maric I, et al. T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood. 2016;128(13):1688–1700. doi: 10.1182/blood-2016-04-711903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fan F, Zhao W, Liu J, et al. Durable remissions with BCMA-specific chimeric antigen receptor (CAR)-modified T cells in patients with refractory/relapsed multiple myeloma. J. Clin. Oncol. 2017;35(Suppl. 18):LBA3001–LBA3001. [Google Scholar]; • Gives information that LCAR-B38M chimeric antigen receptor T-cell therapy is an innovative and highly effective treatment for MM.
- 66.Cohen AD, Garfall AL, Stadtmauer EA, et al. B-cell maturation antigen (BCMA)-specific chimeric antigen receptor T cells (CART-BCMA) for multiple myeloma (MM): initial safety and efficacy from a Phase I study. Blood. 2016;128(22):1147–1147. [Google Scholar]
- 67.Berdeja J, Lin Y, Raje N, et al. Clinical remissions and limited toxicity in a first-in-human multicenter study of bb2121, a novel anti-BCMA CAR T cell therapy for relapsed/refractory multiple myeloma. Eur. J. Cancer. 2016;69:S5. [Google Scholar]
- 68.Guo B, Chen M, Han Q, et al. CD138-directed adoptive immunotherapy of chimeric antigen receptor (CAR)-modified T cells for multiple myeloma. J. Cell. Immunother. 2016;2(1):28–35. [Google Scholar]
- 69.Ramos CA, Savoldo B, Torrano V, et al. Clinical responses with T lymphocytes targeting malignancy-associated kappa light chains. J. Clin. Invest. 2016;126(7):2588–2596. doi: 10.1172/JCI86000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Garfall AL, Stadtmauer EA, Maus MV, et al. Pilot study of anti-CD19 chimeric antigen receptor T cells (CTL019) in conjunction with salvage autologous stem cell transplantation for advanced multiple myeloma. Blood. 2016;128(22):974–974. [Google Scholar]
- 71.Carpenter RO, Evbuomwan MO, Pittaluga S, et al. B-cell maturation antigen is a promising target for adoptive T-cell therapy of multiple myeloma. Clin. Cancer Res. 2013;19(8):2048–2060. doi: 10.1158/1078-0432.CCR-12-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Garfall AL, Lancaster E, Stadtmauer EA, et al. Posterior reversible encephalopathy syndrome (PRES) after infusion of anti-Bcma CAR T cells (CART-BCMA) for multiple myeloma: successful treatment with cyclophosphamide. Blood. 2016;128(22):5702. [Google Scholar]
- 73.Hajek R, Okubote SA, Svachova H. Myeloma stem cell concepts, heterogeneity and plasticity of multiple myeloma. Br. J. Haematol. 2013;163(5):551–564. doi: 10.1111/bjh.12563. [DOI] [PubMed] [Google Scholar]
- 74.Garfall AL, Maus MV, Hwang W-T, et al. Chimeric antigen receptor T cells against CD19 for multiple myeloma. N. Engl. J. Med. 2015;373(11):1040–1047. doi: 10.1056/NEJMoa1504542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baz R, Fanning S, Kunkel L, et al. Combination of rituximab and oral melphalan and prednisone in newly diagnosed multiple myeloma. Leukemia & lymphoma. 2007;48(12):2338–2344.. doi: 10.1080/10428190701644330. [DOI] [PubMed] [Google Scholar]
- 76.Rasche L, Dubljevic V, Lapa C, et al. Anti-GRP78 monoclonal antibody PAT-SM6 in refractory and extramedullary multiple myeloma: Preclinical and clinical evidence for a combinatorial strategy with novel agents. Haematologica. 2015;100:520. [Google Scholar]
- 77.Iida S, Ogiya D, Abe Y, et al. Dose-escalation study of tabalumab with bortezomib and dexamethasone in Japanese patients with multiple myeloma. Cancer Sci. 2016;107(9):1281–1289. doi: 10.1111/cas.13000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lonial S, Dimopoulos MA, Palumbo A, et al. ELOQUENT-2: a Phase III, randomized, open-label study of lenalidomide (Len)/dexamethasone (dex) with/without elotuzumab (Elo) in patients (pts) with relapsed/refractory multiple myeloma (RRMM) J. Clin. Oncol. 2015;33(15_Suppl.):8508–8508. [Google Scholar]
- 79.Palumbo A, Chanan-Khan AAA, Weisel K, et al. Phase III randomized controlled study of daratumumab, bortezomib, and dexamethasone (DVd) versus bortezomib and dexamethasone (Vd) in patients (pts) with relapsed or refractory multiple myeloma (RRMM): CASTOR study. J. Clin. Oncol. 2016;34(18_Suppl.):LBA4–LBA. [Google Scholar]; • This randomized controlled trial advocates the addition of daratumumab to bortezomib and dexamethasone should be considered a new standard of care for relapsed or refractory MM patients currently receiving bortezomib and dexamethasone alone.
- 80.Dimopoulos M, Oriol A, Nahi H, et al. An open-label, randomised Phase III study of daratumumab, lenalidomide, and dexamethasone (DRD) versus lenalidomide and dexamethasone (RD) in relapsed or refractory multiple myeloma (RRMM): POLLUX. Haematologica. 2016;101(Suppl. 1):342. [Google Scholar]; •• Elaborates the role of antibody-based combination therapy (daratumumab, lenalidomide and dexamethasone) in the treatment of relapsed or refractory MM.