Abstract
Study Design:
Narrative review.
Objectives:
The etiology of adjacent segment degeneration (ASDeg) and adjacent segment disease (ASDz) after lumbar interbody fusion (LIF) remains controversial. The aim of this narrative review was to provide an evidence-based analysis of the peer-reviewed literature on clinical studies of ASDeg and ASDz after LIF.
Methods:
A review was performed utilizing Medline, Embase, and Cochrane databases. Two reviewers independently extracted relevant data from each included study. Statistical comparisons were made when appropriate.
Results:
Nine articles that matched the inclusion and exclusion criteria were included. All the studies were Level III and retrospective. MINORS scores ranged from 9.5 to 13. Clinical outcomes were assessed in all 9 studies, but only 6 studies used validated outcomes measures. Only 6 studies reported values for both ASDeg and ASDz. ASDeg alone was reported in 3 studies. Due to the variability in the criteria for designation as ASDz (different radiographic modalities) and ASDeg (different outcomes measures), we were unable to calculate frequency-weighted mean values or compare the various surgical techniques.
Conclusions:
This review highlights the various limitations of the current literature on ASDeg and ASDz after lumbar fusion, specifically the absence of a rigorous definition and classification system and an extraordinary heterogeneity in methodology. There needs to be a fundamental shift in the current ASDeg and ASDz research landscape, toward a consensus, so that the high-level clinical research that is essential for treatment of spinal pathology may become available.
Keywords: LIF, adjacent segment degeneration, adjacent segment disease, fusion, lumbar
Introduction
Briggs and Milligan first described lumbar interbody fusion (LIF) for the surgical treatment of chronic low back pain 70 years ago. Although there is considerable debate over the indications for lumbar fusion, it remains the gold standard surgical treatment for patients who fail nonoperative methods in many clinical scenarios.1–4
Adjacent segment degeneration (ASDeg) is defined as radiographic degenerative changes at a spinal level immediately cranial or caudal to the site of a previous fusion procedure. ASDeg can progress to adjacent segment disease (ASDz), a clinical phenomenon characterized by the presentation of new symptoms referable to the adjacent level, presumably related to the degenerative changes.5 Although ASDeg and ASDz are sometimes used as outcome measures of LIF, their etiology remains controversial. One theory holds that adjacent segment pathologies, like ASDeg and ASDz, are simply reflections of the natural history of lumbar degenerative disease, which is often characterized by desiccation of all lumbar segments, and are therefore inevitable. Others cite biomechanical findings to argue that LIF results in increased motion, intradiscal pressure, and strain adjacent to the fusion, leading to an increased risk of ASDeg and ASDz.5–7
Resolving this debate has proven exceedingly difficult due to the challenges of studying LIF in the clinical setting. Although spinal fusion is an established and exceedingly common procedure,1,8 the steady evolution of surgical techniques and the availability of a myriad of graft materials, cage designs, and plate fixation systems have given rise to considerable variability between treatment methods. In addition, the current literature on adjacent segment pathology suffers from the absence of a universally accepted radiographic modality for diagnosis of ASDeg or validated outcome instrument for diagnosis of ASDz.9–11 Because of this heterogeneity, estimates of ASDz incidence after lumbar fusion range from 1.9% to 30.3%.12,13 One systematic review of ASDeg reported a range of incidence from 8% to 100%.14
As a result of these barriers to clinical research, there is virtually no high-level evidence on ASDeg and ASDz after LIF. The purpose of this narrative review was to analyze the existing literature to determine if there are sufficient similarities among studies to support a meta-analysis.
Materials and Methods
Literature Search
A systematic search of PubMed, Embase, and Cochrane Reviews for literature published through June 2013 was done on July 31, 2013. The search terms for PubMed and Embase are presented in Tables 1 and Table 2. The PRISMA flow diagram is presented in Figure 1. Search results were limited to full text articles with a minimum follow-up of 1 year written in the English language. All abstracts were independently reviewed by 2 clinical research fellows and a fellowship-trained orthopedic spine surgeon. The following study designs were excluded from this analysis: meta-analyses, systematic reviews, and review articles. Articles focused on the following topics were also excluded: biomechanics analyses (cadaveric, in vitro), animal studies, trauma, and tumor studies.
Table 1.
Medline Literature Search.
| Search | Query Input |
|---|---|
| #1 | Spinal Fusion [MeSH] or “Spinal Fusion” |
| #2 | Lumbar [MeSH] or Lumbar |
| #3 | 1 AND 2 |
| #4 | ALIF or “Anterior Lumbar Interbody Fusion” |
| #5 | PLIF or “Posterior Lumbar Interbody Fusion” |
| #6 | PLF or “Posterior Lumbar Fusion” |
| #7 | TLIF or “Transforaminal Lumbar Interbody Fusion” |
| #8 | XLIF or “Extreme Lateral Interbody Fusion” |
| #9 | 4 OR 5 OR 6 OR 7 OR 8 |
| #10 | 3 OR 9 |
| #11 | “adjacent segment” or “adjacent level” |
| #12 | 10 OR 11 |
Table 2.
Embase literature Search.
| Search | Query Input |
|---|---|
| #1 | spine fusion.mp. or spine fusion/ |
| #2 | lumbar.mp. or exp lumbar spine/ or exp lumbar disk/ or exp lumbar spinal cord/ |
| #3 | (ALIF or “Anterior Lumbar Interbody Fusion”).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] |
| #4 | (PLIF or “Posterior Lumbar Interbody Fusion”).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] |
| #5 | (PLF or “Posterior Lumbar Fusion”).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] |
| #6 | (TLIF or Transforaminal Lumbar Interbody Fusion).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] |
| #7 | (XLIF or “Extreme Lateral Interbody Fusion”).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] |
| #8 | (“Adjacent Segment” or “Adjacent Level”).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] |
| #9 | 1 AND 2 |
| #10 | 3 OR 4 OR 5 OR 6 OR 7 OR 8 |
| #11 | 8 OR 9 |
| #12 | 10 AND 7 |
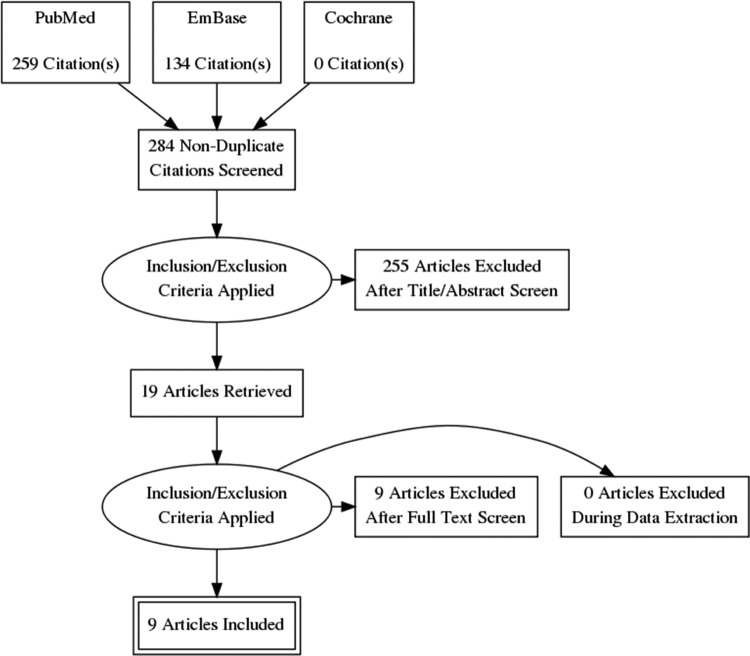
Figure 1.
PRISMA flowsheet.
Articles (n = 284) from the primary literature search were obtained. Of the original 284 articles, 265 were excluded based on the abstract because of study design or irrelevance to the topic in question. The full texts of the remaining 19 articles were then retrieved and further examined by 3 reviewers (NNT, LAP, and DRL). After critical examination of the full text of the remaining 19 articles, 10 additional articles were excluded, leaving 9 articles to be included in this review. Relevant data was identified and extracted from the full text of all remaining articles (Table 3).
Table 3.
Summary of Literature.
| Study | Number of Patients (Males/Females) | Age (Average) | Surgeries Performed | Follow-up: Months, Average (Range) | Clinical Assessment Tools | Radiographic Modality | ASDegeneration (% Patients) | ASDisease (% Patients) |
|---|---|---|---|---|---|---|---|---|
| Bae et al12 | 103 (39/94) | 48.5 | Mini-ALIF; mini-TLIF | 51.9 (36-94) | Visual Analog Scale, ODI, patient’s return to work status | CT | 10.7 | 1.9 |
| Chou et al25 | 32 (20/12) | 70.5 | PLF | 56 (48-66) | Tolerance of walking claudication and the severity of back pain or radicular pain | X-ray | 18.8 | 12.5 |
| Kim et al17 | 94 (23/71) | 50.2 | ALIF and PLIF | 70 (62-86) | VAS and ODI scores | MRI | 75.7 | 18.1 |
| Axelsson et al19 | 9 (3/6) | 45 | PLF with/without instrumentation and ALIF | 60 | Pain Scale (3 options) | X-ray | 22.2 | 11.1 |
| Rahm et al18 | 102 (69/33) | 54 | Anterior fusion with a pararectal approach; AP fusion with a ventral pararectal approach | 13.8 | ODI/Patient Satisfaction Scores | X-ray | 26.5 | 27.5 |
| Wai et al22 | 39 (19/20) | 58.3 | ALIF | 246 (240-306) | Low Back Outcome Scale | MRI | 74.3 | NA |
| Okuda et al23 | 87 (38/49) | 64 | PLIF | 43 (24-78) | Japanese Orthopedic Association score | MRI and CT | 67 | NA |
| Min et al21 | 48 (14/34) | NA | ALIF, PLIF, TLIF | 44.6 (24-68) | Japanese Orthopedic Association score | X-ray | 62.5 | NA |
| Gillet et al24 | 106 (35/71) | NA | PLF | 60 | Reoperation | Did not specify | 41 | 20 |
Abbreviations: ALIF, anterior lumbar interbody fusion; TLIF, transforaminal lumbar interbody fusion; VAS, Visual Analog Scale; ODI, Oswestry Disability Index; CT, computed tomography; PLF, posterior lumbar fusion; PLIF, posterior lumbar interbody fusion; MRI, magnetic resonance imaging.
Level of Evidence
The criteria outlined by Wright et al were used to evaluate the relative merit of the studied included in this review.15 In addition, the MINORS criteria outlined by Slim et al were used to evaluate the studies.16 Each article was independently graded by 2 authors. Any interreviewer variation, defined as difference in study level or difference in MINORS score >2, would be resolved by a third reviewer.
Results
Nine articles that matched the inclusion and exclusion criteria were included (Table 3).11,12,17–23 All of the studies were determined to be Level 3 evidence and retrospective, and the average MINORS scores ranged from 9.5 to 13 (Table 4). There were a multitude of clinical and radiographic initial presentations that were reported (isthmic spondylolisthesis, degenerative spondylolisthesis, degenerative disc pain, etc).
Table 4.
Summary of Level of Evidence and MINORS Scores.
| Study | Level of Evidence11 | MINORS Score9 |
|---|---|---|
| Bae et al12 | Level III (Retrospective) | 12.5 |
| Chou et al25 | Level III (Retrospective) | 9.5 |
| Kim et al17 | Level III (Retrospective) | 13 |
| Axelsson et al19 | Level III (Retrospective) | 11 |
| Rahm et al18 | Level III (Retrospective) | 10 |
| Wai et al22 | Level III (Retrospective) | 11 |
| Okuda et al23 | Level III (Retrospective) | 10 |
| Min et al21 | Level III (Retrospective) | 11.5 |
| Gillet et al24 | Level III (Retrospective) | 11 |
Demographics
In total, 625 patients were included in the 9 studies with an average of 69 patients per study (range = 9-133). All 9 studies provided demographic data on sex, leading to a total of 254 males and 371 females. Seven studies provided age. The frequency-weighted mean age for those 7 studies was 43.4 (range 45-70.5). The frequency-weighted average follow-up was 60.1 months with a range from 13.8 to 246.
Surgical Technique
Six studies specifically analyzed 1- or 2-level lumbar fusion,12,17–19,21,23 and 3 studies pooled data on several fusion lengths, ranging from 1 to 4 levels.22,24,25 Plate fixation was used in one of the articles, accounting for 13% of surgeries. Pedicle screw was used in 7 of the articles, accounting for 87% of surgeries.
Two studies reported using only posterior lumbar fusion (PLF) for fusion,24,25 3 studies reported using only anterior lumbar interbody fusion (ALIF),17,18,22 one study reported using only posterior lumbar interbody fusion (PLIF),23 and 3 studies were either nonspecific or reported multiple surgical techniques.12,19,21 Because of the variability in measuring ASDeg and ASDz, we were unable to compare incidence for the various surgical techniques.
Outcome Assessment
Clinical outcomes were assessed in all 9 studies. Six used validated outcomes measures, including the visual analog scale,12 Oswestry Disability Index,17,18 Low Back Outcome Scale,22 and Japanese Orthopedic Association score.11,21 The remaining 3 studies reported tolerance of walking,25 pain scale,19 and reoperation.24
Radiographic evaluation was reported in all 9 of the studies. Of the modalities employed, plain radiographs were used in 5 studies,18,19,21,25 with computed tomography in 2 studies11,12 and magnetic resonance imaging in 4 studies.11,12,17,22 One study did not specify radiographic modality.24
Outcomes: ASDeg and ASDz
Only 6 articles reported incidence for both ASDeg and ASDz.12,17–19,24,25 Adjacent segment degeneration alone was reported in 3 studies.21–23 However, due to the variability in criteria for designation as ASDz or ASDeg, we were unable to calculate frequency-weighted mean values.
Discussion
Long-term follow-up studies on LIF have described radiographic deterioration of discs adjacent to the level of index surgery.5,26 ,27 Adjacent segment pathology is differentiated in the literature into radiographic degeneration (ASDeg) and the associated clinical entity (ASDz).28 Unfortunately, there is no consensus on the definitions or classification systems for ASDeg or ASDz. Nevertheless, this distinction is useful for surgical decision making and patient counseling regarding the likelihood of the need for reoperation.
The relationship between ASDeg and ASDz after LIF remains an important topic of research interest. There is some data indicating that the severity of ASDeg might predict clinical outcome, specifically ASDz, but the evidence is limited.29 Other studies have found no association between the degree of ASDeg and clinical or functional outcomes, and some suggest that routine radiographic follow-up has limited utility.14,23,30–33 Rigorous definitions and classification methods for ASDeg and ASDz are required before high-quality studies can address this question with any certainty.
The central debate revolves around the etiology of adjacent segment pathology, specifically whether ASDeg and ASDz are part of the natural history of the disease or induced by biomechanical adjustments postsurgery. Proponents of the natural history theory argue that lumbar degenerative disease is characterized by gradual desiccation of all lumbar discs, and therefore deterioration would occur even without surgery. The natural history argument is supported indirectly by evidence that many asymptomatic patients exhibit progressive abnormal radiographic findings. One study found that disc degeneration progresses in 41% of asymptomatic patients and another demonstrated that 57% of asymptomatic patients over 60 years of age have abnormal findings on magnetic resonance imaging scans of the lumbar spine.34,35 In addition, Wai et al determined in a series of 39 patients that the majority of degenerative changes occurred homogenously across lumbar levels, suggesting that deterioration is determined by characteristics specific to the patient.22 Similarly, Pisellé et al found that in patients with degenerated adjacent disc, 50% of other levels also exhibited degeneration. In patients with normal adjacent discs, only 8.6% of the remaining discs had degeneration.36 Evidence of a genetic predisposition for lumbar degenerative disease offers further support for this argument. Twin studies attribute 26% to 76% of the variability in disease incidence to genetic factors.37 Although many proponents of the natural history theory do not fully dismiss the biomechanical explanation of ASDeg and ASDz, they argue that it is negligible.
This argument is complicated by evidence, albeit limited and low level, that lumbar fusion patients have an elevated rate of ASDeg and ASDz over nonsurgical controls.38 The biomechanical argument supplies an attractive explanation for this possible elevation of ASDeg and ASDz incidence. Numerous biomechanical studies using animal and human models demonstrate that lumbar fusion can result in pressure, force, and motion adjustments at adjacent levels.5–7,19,39–43 However, these studies only demonstrate changes in biomechanical forces, and although various animal models have demonstrated ASDeg and ASDz after fusion, there is no quality clinical evidence that biomechanical changes directly cause radiographic (ASDeg) and clinical (ASDz) deterioration.44–46
Furthermore, both cadaveric and animal studies are severely limited in their ability to reproduce the in vivo properties of the human spine, and some authors argue that this results in an overestimation of mechanical alterations. In one biomechanical study, Rohlmann et al concluded that interspecimen differences and the complex loading conditions that do not reproduce the in vivo environment best explain the observed changes in pressures.47 Clinical studies have also challenged the biomechanical argument. In a radiographic study of hypermobility after lumbar spine fusion, Axelsson et al found no increase in mean mobility 5 years after surgery and no correlation between mobility and clinical outcomes.20 However, although this study is clinical, it suffers from a small sample size (n = 9) and other limitations. Because of the absence of high-quality clinical research, biomechanical studies will remain central to the debate, despite their obvious limitations.
Finally, this argument is even further complicated by the multitude of surgical techniques for LIF currently in practice, including ALIF, TLIF (transforaminal lumbar interbody fusion), XLIF (extreme lateral interbody fusion), PLF, and PLIF. Some argue that ALIF allows for preservation of the posterior ligaments and soft tissue, which could further prevent the likelihood of ASDeg and ASDz. However, there have been no consistent findings in the literature substantiating this claim or that any of the LIF techniques is superior with respect to ASDeg and ASDz.
There are many proposed risk factors for ASDeg and ASDz, including age, instrumentation, fusion type, fusion length, and degree of lumbar lordosis. The studies in this review addressed some of these risk factors, though pervasive covariability and low sample sizes limit their generalizability and conclusiveness.
There is evidence in the literature, including studies in this review, that the degree of lumbar lordosis is an important risk factor for adjacent segment pathology.12,17,21,48 Bae et al demonstrated that abnormal postoperative segmental lordosis showed a significant correlation with ASDeg (P = .008).12 A case-control study of 51 patients demonstrated that those who went on to develop ASDeg had a postoperative mean lordosis of −15.3° compared to a mean lordosis of −23.4° in controls.48 There is also evidence that the lordotic angle may be related to severity of disease. In patients undergoing L4-L5 fusion, Kim et al found that ASDeg patients had a significantly higher lordotic angle (ie, >20°) compared to ASDz patients.17 In a study of 48 patients undergoing L4-L5 fusion, Min et al observed that the mean difference between pre- and postoperative lordosis was associated with ASDeg, suggesting that maintaining the preoperative lordosis may protect against the development of ASDeg.21 It is important to note that many other studies have not reproduced these findings to date.
The number of fused levels is another proposed risk factor for ASDeg and ASDz, and there is evidence that patients with multilevel fusions are at 2 to 3 times greater risk for ASDz.49,50 However, in a study of 215 patients, Ghiselli et al reported that single-level fusion patients are up to 3 times more likely to develop ASDz.27 Six studies in this review included multilevel fusions, and 3 of those evaluated the risk associated with multilevel fusions. Chou et al found a trend toward higher incidence of ASDeg in the multilevel fusion group, but this was not statistically significant (P = .79).25 Rahm et al detected a similar correlation between the number of levels and ASDeg, but this was also not significant due to a lack of power.18 Gillet et al observed reoperation rates for single, double, and 3- to 4-level fusions of 11%, 27%, and 33%, respectively.24 Taken together with the existing literature, it seems likely that there is an increased risk of ASDz with multilevel fusions, but there is need for higher quality studies. The correlation between number of fusion levels and ASDeg remains unclear.
This review is most useful to the extent that it highlights the current limitations in the literature. For one, it illustrates the low quality of evidence currently available. No Level I or Level II studies met the search criteria: the review was limited to studies with Level III evidence. Furthermore, this review found no studies with MINORS criteria scores greater than 13, the major limiting factor being the retrospective nature of all the studies that met the inclusion criteria.
The review also highlights some of the practical barriers to quality clinical research. The heterogeneity of approaches used to detect ASDeg and ASDz is the most important. For example, while all 9 of the studies in this review analyzed ASDz incidence, they used 10 different clinical assessment tools in 8 combinations. These measures ranged from reoperation to an unvalidated pain scale. Only 2 pairs of studies used the same metric to define ASDz. Similar variation in evaluation systems was evident in the analysis of ASDeg. This inconsistency is common throughout the literature and poses a major barrier to high-quality research.
Indeed, the defining feature of this narrative review was the variability between studies. Surgery type, number and location of fused levels, screw type, cage type, and graft material differed considerably between (and within) studies, when provided at all. For example, average follow-up time varied from 3 to 246 months.20,22 Such inconsistencies introduce an challenging mass of covariables. This reality, considered in the absence of rigorous definition and classification systems, makes it impractical to synthesize the existing literature with any confidence. The extraordinary variability between studies also precludes a more sophisticated statistical approach (ie, meta-analysis).
Conclusion
Despite the high success rate of LIF, follow-up has identified ASDeg and ASDz as potential postoperative complications. The literature is currently divided on whether adjacent segment pathology represents the natural history of the disease or results from surgery-induced biomechanical stresses. This review highlights the various limitations of the current literature on ASDeg and ASDz after lumbar fusion, specifically the absence of a rigorous definition and classification system and an extraordinary heterogeneity in methodology. There needs to be a fundamental shift in the current ASDeg and ASDz research landscape, toward a consensus, so that the high-level clinical research that is essential for treatment of spinal pathology may become available. This shift must begin with the development of a rigorous definition and classification system for ASDeg and ASDz. It will also require more consistent methodology, something that multi-surgeon collaboration could help achieve.
Footnotes
Authors’ Note: Investigation performed at the Department of Orthopaedic Surgery, Hospital for Special Surgery, New York, NY, USA.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Melancia JL, Francisco FA, Antunes JL. Spinal stenosis. Handb Clin Neurol. 2014;119:541–549. [DOI] [PubMed] [Google Scholar]
- 2. Briggs H, Milligan PR. Chip fusion of the low back following exploration of the spinal canal. J Bone Jt Surg. 1944;26:125–130. [Google Scholar]
- 3. Cole CD, McCall TD, Schmidt MH, Dailey AT. Comparison of low back fusion techniques: transforaminal lumbar interbody fusion (TLIF) or posterior lumbar interbody fusion (PLIF) approaches. Curr Rev Musculoskeletal Med. 2009;2:118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Christensen FB. Outcome in relation to surgical methods, choice of implant and postoperative rehabilitation. Acta Orthop Scand Suppl. 2004;75:2–43. [PubMed] [Google Scholar]
- 5. Radcliff KE, Kepler CK, Jakoi A, et al. Adjacent segment disease in the lumbar spine following different treatment interventions. Spine J. 2013;13:1339–1349. [DOI] [PubMed] [Google Scholar]
- 6. Abode-Iyama K, Kim SB, Grosland N, et al. Spinal motion and intradiscal pressure measurements before and after spine instrumentation with titanium or PEEK rods. J Clin Neurosci. 2014;21:651–655. [DOI] [PubMed] [Google Scholar]
- 7. Schlegel JD, Smith JA, Schleusener RL. Lumbar motion segment pathology adjacent to the thoracolumbar, lumbar and lumbosacral fusions. Spine (Phila Pa 1976). 1996;21:970–981. [DOI] [PubMed] [Google Scholar]
- 8. Rajaee SS, Bae HW, Kanim LE, Delamarter RB. Spinal fusion in the United States: analysis of trends from 1998 to 2008. Spine (Phila Pa 1976). 2012;37:67–76. [DOI] [PubMed] [Google Scholar]
- 9. Kraemer P, Fehlings MG, Hashimoto R, et al. A systematic review of definitions and classification systems of adjacent segment pathology. Spine (Phila Pa 1976). 2012;37(22 suppl):S31–S39. [DOI] [PubMed] [Google Scholar]
- 10. Mok JM, Cloyd JM, Bradford DS, et al. Reoperation after primary fusion for adult spinal deformity: rate, reason and timing. Spine (Phila Pa 1976). 2009;34:832–839. [DOI] [PubMed] [Google Scholar]
- 11. Lee CS, Hwang CJ, Lee SW, et al. Risk factors for adjacent segment disease after lumbar fusion. Eur Spine J. 2009;18:1637–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bae JS, Lee SH, Kim JS, Jung B, Choi G. Adjacent segment degeneration after lumbar interbody fusion with percutaneous pedical screw fixation for adult low-grade isthmic spondylolisthesis: minimum 3 years follow-up. Neurosurgery. 2010;67:1600–1607. [DOI] [PubMed] [Google Scholar]
- 13. Cheh G, Bridwell KH, Lenke LG, et al. Adjacent segment disease following lumbar/thoracolumbar fusion with pedicle screw instrumentation: a minimum 5-year follow-up. Spine (Phila Pa 1976). 2007;32:2253–2257. [DOI] [PubMed] [Google Scholar]
- 14. Park P, Garton HJ, Gala VC, Hoff JT, McGillicuddy JE. Adjacent segment disease after lumbar or lumbosacral fusion: review of the literature. Spine (Phila Pa 1976). 2004;29:1938–1944. [DOI] [PubMed] [Google Scholar]
- 15. Wright JG, Swiontkowski M, Heckman JD. Levels of evidence. J Bone Joint Surg Br. 2006;88:1264. [DOI] [PubMed] [Google Scholar]
- 16. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712–716. [DOI] [PubMed] [Google Scholar]
- 17. Kim KH, Lee SH, Shim CS, et al. Adjacent segment disease after interbody fusion and pedicle screw fixations for isolated L4-L5 Spondylolisthesis: a minimum five-year follow-up. Spine (Phila Pa 1976). 2010;35:625–634. [DOI] [PubMed] [Google Scholar]
- 18. Rahm MD, Hall BB. Adjacent-segment degeneration after lumbar fusion with instrumentation: a retrospective study. J Spinal Disord. 1996;9:392–400. [PubMed] [Google Scholar]
- 19. Axelsson P, Johnsson R, Strömqvist B. Adjacent segment hypermobility after lumbar spine fusion: no association with progressive degeneration of the segment 5 years after surgery. Acta Orthop. 2007;78:834–839. [DOI] [PubMed] [Google Scholar]
- 20. Ghiselli G, Wang JC, Hsu WK, Dawson EG. L5-S1 segment survivorship and clinical outcome analysis after L4-L5 isolated fusion. Spine (Phila Pa 1976). 2003;28:1275–1280. [DOI] [PubMed] [Google Scholar]
- 21. Min JH, Jang JS, Jung BJ, et al. The clinical characteristics and risk factors for the adjacent segment degeneration in instrumented lumbar fusion. J Spinal Disord Tech. 2008;21:305–309. [DOI] [PubMed] [Google Scholar]
- 22. Wai EK, Santos ER, Morcom RA, Fraser RD. Magnetic resonance imaging 20 years after anterior lumbar interbody fusion. Spine (Phila Pa 1976). 2006;31:1952–1956. [DOI] [PubMed] [Google Scholar]
- 23. Okuda S, Iwasaki M, Miyauchi A, Aono H, Morita M, Yamamoto T. Risk factors for adjacent segment degeneration after PLIF. Spine (Phila Pa 1976). 2004;29:1535–1540. [DOI] [PubMed] [Google Scholar]
- 24. Gillet P. The fate of the adjacent motion segments after lumbar fusion. J Spinal Disord Tech. 2003;16:338–345. [DOI] [PubMed] [Google Scholar]
- 25. Chou WY, Hsu CJ, Chang WN, Wong CY. Adjacent segment degeneration after lumbar spinal posterolateral fusion with instrumentation in elderly patients. Arch Orthop Trauma Surg. 2002;122:39–43. [DOI] [PubMed] [Google Scholar]
- 26. Choi KC, Kim JS, Shim HK, Ahn Y, Lee SH. Changes in the adjacent segment 10 years after anterior lumbar interbody fusion for low-grade isthmic spondylolisthesis. Clin Orthop Relat Res. 2014;472:1845–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ghiselli G, Wang JC, Bhatia NN, Hsu WK, Dawson EG. Adjacent segment degeneration in the lumbar spine. J Bone Joint Surg Am. 2004;86-A:1497–1503. [DOI] [PubMed] [Google Scholar]
- 28. Hilibrand AS, Robbins M. Adjacent segment degeneration and adjacent segment disease: the consequences of spinal fusion? Spine J. 2004;4(6 suppl):190S–194S. [DOI] [PubMed] [Google Scholar]
- 29. Yang JY, Lee JK, Song HS. The impact of adjacent segment degeneration on the clinical outcome after lumbar spinal fusion. Spine (Phila Pa 1976). 2008;33:503–507. [DOI] [PubMed] [Google Scholar]
- 30. Ha KY, Son JM, Im JH, Oh IS. Risk factors for adjacent segment degeneration after surgical correction of degenerative lumbar scoliosis. Indian J Orthop. 2013;47:346–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yu CH, Lee JE, Yang JJ, Chang BS, Lee CK. Adjacent segment degeneration after single-level PLIF: comparison between spondylolytic spondylolisthesis, degenerative spondylolisthesis and spinal stenosis. Asian Spine J. 2011;5:82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yamashita T, Steinmetz MP, Lieberman IH, Modic MT, Mroz TE. The utility of repeated postoperative radiographs after lumbar instrumented fusion for degenerative lumbar spine. Spine (Phila Pa 1976). 2011;36:1955–1960. [DOI] [PubMed] [Google Scholar]
- 33. Frymoyer JW, Hanley EN, Jr, Howe J, Kuhlmann D, Matteri RE. A comparison of radiographic findings in fusion and nonfusion patients ten or more years following lumbar disc surgery. Spine (Phila Pa 1976). 1979;4:435–440. [DOI] [PubMed] [Google Scholar]
- 34. Boden SD, Davis DO, Dina TS, Patronas NJ, Wiesel SW. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic patients. A prospective investigation. J Bone Joint Surg Am. 1990;72:403–408. [PubMed] [Google Scholar]
- 35. Elfering A, Semmer N, Birkhofer D, Zanetti M, Hodler J, Boos N. Risk factors for lumbar disc degeneration: a 5-year prospective MRI study in asymptomatic individuals. Spine (Phila Pa 1976). 2002;27:125–134. [DOI] [PubMed] [Google Scholar]
- 36. Pisellé F, Hernández A, Vidal X, Minguell J, Martínez C, Villanueva C. Radiologic assessment of all unfused lumbar segments 7.5 years after instrumented posterior spinal fusion. Spine (Phila Pa 1976). 2007;32:574–579. [DOI] [PubMed] [Google Scholar]
- 37. Battié MC, Videman T, Kaprio J, et al. The Twin Spine Study: contributions to a changing view of disc degeneration. Spine J. 2009;9:47–59. [DOI] [PubMed] [Google Scholar]
- 38. Lee MJ, Dettori JR, Standaert CJ, Brodt ED, Chapman JR. The natural history of degeneration of the lumbar and cervical spines: a systematic review. Spine (Phila Pa 1976). 2012;37(22 suppl): S18–S30. [DOI] [PubMed] [Google Scholar]
- 39. Dekutoski MB, Schendel MJ, Ogilvie JW, Olsewski JM, Wallace LJ, Lewis JL. Comparison of in vivo and in vitro adjacent segment motion after lumbar fusion. Spine (Phila Pa 1976). 1994;19:1745–1751. [DOI] [PubMed] [Google Scholar]
- 40. Ha KY, Schendel MJ, Lewis JL, Ogilvie JW. Effect of immobilization and configuration on lumbar adjacent-segment biomechanics. J Spinal Disord. 1993;6:99–105. [PubMed] [Google Scholar]
- 41. Olsewski JM, Schendel MJ, Wallace LJ, Ogilvie JW, Gundry CR. Magnetic resonance imaging and biological changes in injured intervertebral discs under normal and increased mechanical demands. Spine (Phila Pa 1976). 1996;21:1945–1951. [DOI] [PubMed] [Google Scholar]
- 42. Chen CS, Cheng CK, Liu CL, Lo WH. Stress analysis of the disc adjacent to interbody fusion in lumbar spine. Med Eng Phys. 2001;23:483–491. [DOI] [PubMed] [Google Scholar]
- 43. Eck JC, Humphreys SC, Hodges SD. Adjacent-segment degeneration after lumbar fusion: a review of clinical, biomechanical, and radiologic studies. Am J Orthop (Belle Mead NJ). 1999;28:336–340. [PubMed] [Google Scholar]
- 44. Hoogendoorn RJ, Helder MN, Wuisman PI, Bank RA, Everts VE, Smit TH. Adjacent segment degeneration: observations in a goat spinal fusion study. Spine (Phila Pa 1976). 2008;33:1337–1343. [DOI] [PubMed] [Google Scholar]
- 45. Lotz JC, Colliou OK, Chin JR, Duncan NA, Liebenberg E. Compression-induced degeneration of the intervertebral disc: an in vivo mouse model and finite-element study. Spine (Phila Pa 1976). 1998;23:2493–2506. [DOI] [PubMed] [Google Scholar]
- 46. Phillips FM, Reuben J, Wetzel FT. Intervertebral disc degeneration adjacent to a lumbar fusion. J Bone Joint Surg Br. 2002;84:289–294. [DOI] [PubMed] [Google Scholar]
- 47. Rohlmann A, Neller S, Bergmann G, Graichen F, Claes L, Wilke HJ. Effect of an internal fixator and a bone graft on intersegmental spinal motion and intradiscal pressure in the adjacent regions. Eur Spine J. 2001;10:301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Djurasovic MO, Carreon LY, Glassman SD, Dimar JR, 2nd, Puno RM, Johnson JR. Sagittal alignment as a risk factor for adjacent level degeneration: a case-control study. Orthopedics. 2008;31:546. [PubMed] [Google Scholar]
- 49. Ahn DK, Park HS, Choi DJ, Kim KS, Yang SJ. Survival and prognostic analysis of adjacent segments after spinal fusion. Clin Orthop Surg. 2010;2:140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sears WR, Sergides IG, Kazemi N, Smith M, White GJ, Osburg B. Incidence and prevalence of surgery at segments adjacent to a previous posterior lumbar arthrodesis. Spine J. 2011;11:11–20. [DOI] [PubMed] [Google Scholar]