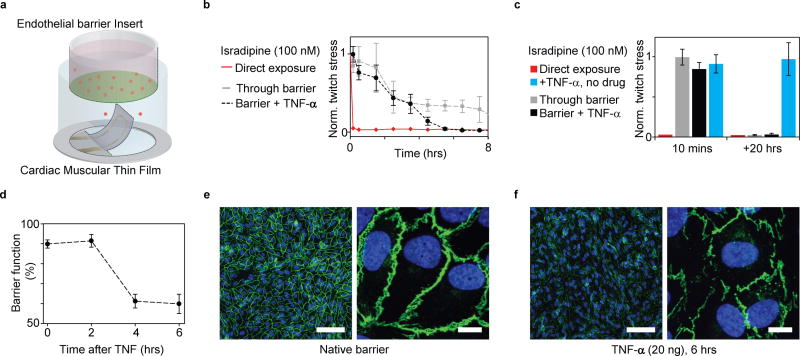
Figure 6. Coupling endothelial barriers with cardiac MTFs to study drug transport.
(a) Schematic illustration of experiments. Endothelial barrier tissues in Transwell® inserts are introduced into the wells of the instrumented cardiac 24-well MTF platform. A cardiac drug (Isradipine) is introduced in the volume shielded by the endothelial barrier. Drug transport across barrier and resultant cardiac effects are recorded in real-time through embedded sensors. (b) Decrease in normalized contractile twitch stress over time. Direct exposure of Isradipine (100 nM) immediately halts contraction (red). Exposure of Isradipine (100 nM) through endothelial barrier inserts (grey line), leads to delayed drug effect, where contraction is still observed after > 7 h exposure. (N=3). Compromising endothelial barrier function by co exposure with TNF-α significantly accelerates drug diffusion, leading to a full effect of the drug after ~5 h. (c) After 20 h full effect of Isradipine was observed for all samples. TNF-α did not directly introduce changes in twitch stress of cardiac tissues. (d) Endothelial tissue barrier function decreases upon exposure to TNF-α (20 ng/ml). Barrier function was evaluated as percent of fluorescent marker (400 Da) contained inside barrier during 20 mins diffusion into reservoir without tracer (e) Confocal microscopy of immunostained intact endothelial barrier. Blue: DAPI nuclei stain, Green: VE-Cadherin, Scale bars left: 100 µm, right: 10 µm (f) Confocal microscopy of immunostained endothelial barrier after exposure to 20 ng/ml TNF-α for 6 h. Blue: DAPI nuclei stain, Green: VE-Cadherin, Scale bars left: 100 µm, right: 10 µm.