Table 1. Optimization of reaction conditions a .
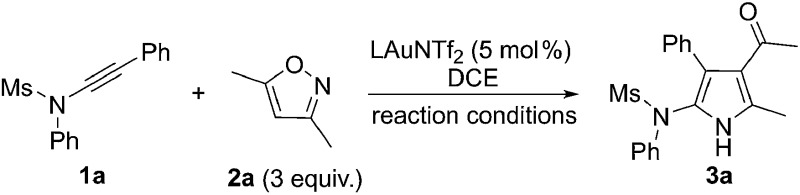
| |||
| Entry | Metal catalyst | Conditions | Yield b (%) |
| 1 | IPrAuNTf2 | DCE, 80 °C, 3 h | 70 |
| 2 | Ph3PAuNTf2 | DCE, 80 °C, 3 h | 69 |
| 3 | Et3PAuNTf2 | DCE, 80 °C, 3 h | 54 |
| 4 | Cy-JohnPhosAuNTf2 | DCE, 80 °C, 3 h | 71 |
| 5 | BrettPhosAuNTf2 | DCE, 80 °C, 12 h | 27 |
| 6 | Au(III) c | DCE, 80 °C, 3 h | 34 |
| 7 | (ArO)3PAuNTf2 d | DCE, 80 °C, 3 h | 95 |
| 8 | AgNTf2 | DCE, 80 °C, 3 h | 50 |
| 9 e | PtCl2 | toluene, 80 °C, 3 h | <5 |
| 10 e | Zn(OTf)2 (10 mol%) | DCE, 80 °C, 3 h | <5 |
| 11 | (ArO)3PAuNTf2 d | DCE, 60 °C, 5 h | 75 |
| 12 f | (ArO)3PAuNTf2 d | DCE, 80 °C, 3 h | 90 |
aReaction conditions: [1a] = 0.05 M; DCE = 1,2-dichloroethane.
bMeasured by 1H NMR using diethyl phthalate as the internal standard.
cDichloro(2-picolinato)gold(iii).
d Ar = 2,4-di-tert-butylphenyl.
e 1a was decomposed.
f2.0 equiv. of 2a was used.
