Abstract
Innervation is a fundamental basis for function and survival of tissues. In the peripheral tissues, degenerative diseases create a neurotoxic metabolic milieu that either causes neurodegeneration or fails to sustain regenerative growth and re-innervation of injured/diseased tissues. Encapsulation of cells producing neurotrophic factors can augment axon growth and neuron survival; however, sustained innervation in vivo requires a combination of factors promoting axon growth and guidance pathway that are released in a tissue-specific context. Using novel encapsulation techniques and genetic tools, we manipulated retinoic acid-generating enzyme aldehyde dehydrogenase 1a1 (Aldh1a1) in adipocytes that is capable of promoting growth and innervation of white adipose tissue (WAT) by sympathetic neurons. Aldh1a1−/− adipocytes secrete molecules that regulate axon-guidance and markedly stimulate neurite outgrowth in vitro and in vivo. Based on studies with natural and synthetic RAR agonists and antagonists, gene microarray and nanostring arrays, we concluded that ephrin A5/ephrin A4 is a downstream pathway regulated by Aldh1a1. Encapsulation of Aldh1a1−/− adipocytes into alginate poly-L-lysine microcapsules induced functional innervation of adipose tissue in obese wild-type mice. We propose that encapsulated Aldh1a1−/− adipocytes could provide a therapeutic solution for the reinnervation of damaged tissues.
INTRODUCTION
Neuronal damage [1, 2] and neurodegeneration [3–5] impair central nervous system (CNS) function, they play critical role in the development and progression of metabolic disorders [6, 7]. Axons provide a template for vascularization [8]. Additionally, axons integrate responses between peripheral tissues and the CNS [9]. Sympathetic innervation of white adipose tissue (WAT) controls essential metabolic processes such as lipolysis and energy dissipation by thermogenic adipocyte [10, 11]. Malfunctions in lipolysis and thermogenesis are associated with obesity [12]. Although the critical role for peripheral innervation in tissue homeostasis is well established, researchers still face considerable challenges in rebuilding axonal circuits after metabolic damage or injuries.
A significant challenge for rebuilding neuronal circuits is the failure of CNS axons to regenerate after injury. Peripheral nervous system (PNS) axons are capable of regeneration [13] and, thus, represent a more viable target for regeneration therapies. Such therapies typically focus on improving neuron survival and enhancing axon growth [14]. Even though PNS nerves can regenerate [15], functional regeneration is often incomplete, due to decreased trophic support from peripheral cells [16]. One way to enhance the growth-supportive milieu for axons is to provide engineered encapsulating cells into preferred target sites. Cell encapsulation appears to be a safe and sustainable approach to deliver desirable compounds, such as NGF or brain-derived neurotrophic factor (BDNF), to the brain and other tissues [17, 18]. Although treatment with neurotrophic factors improves neuronal survival, it lacks other cues that guide axons and enable the formation of functional networks. These cues include axon guidance molecules (for attraction and repulsion), specific proteases, and other regulatory molecules responsible for regulation of G-proteins signaling cascades [8]. Together these cues allow for the formation of specific neuronal networks between peripheral tissues and the CNS. Because it is not conceivable to engineer cells to express all these molecules, it is critical to identify cells and/or regulatory pathways upstream of the production of axon guidance cues.
One such cell type is preadipocytes/fibroblasts. Preadipocytes have been identified as a common environment of peripheral vascular innervation [19]. Preadipocytes also play an important role in intracrine and paracrine regulation. Specifically, preadipocytes produce metabolites of vitamin A, such as retinoic acid (RA) and retinaldehyde (Rald) (Figure 1A) [20]. RA acts as an ligand for the retinoic acid receptor (RAR) and activates transcriptional or translational responses of RAR [21]. This receptor plays an important role in both thermogenesis and differentiation of neurons [21–23]. In preadipocytes and adipocytes, intracellular production of RA regulates adipogenesis and adipokine production [21]. Additionally, RA could potentially regulate de novo neurogenesis from stromal cells in a paracrine fashion [22]. RAR activation in adipocytes depends on the aldehyde dehydrogenase 1 (ALDH1A1) enzyme [20]. Surprisingly, Aldh1a1−/−-deficient mice producing 70% less RA [22] also exhibit increased thermogenesis in WAT [24, 25]. Whether increased thermogenesis in Aldh1a1−/− mice is associated with improved sympathetic innervation it unknown [11]. Here, we identified a role for Aldh1a1−/− preadipocytes in the production of axon guidance molecules in vivo and in vitro and established a connection between increased WAT innervation and metabolic rate in mice engrafted with Aldh1a1−/− adipocytes.
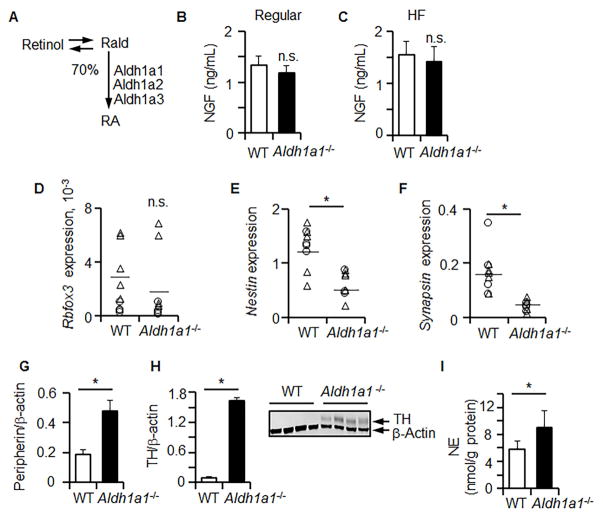
Figure 1. Aldh1a1 deficiency induces innervation in WAT in vivo without an increase in neural precursors.
(A) Schematic of the main processes leading to production of retinoic acid (RA) in WAT. ALDH1A1 produces 70% of RA which activates RAR in adipocytes (100%) [20]. NGF plasma levels were examined by ELISA in WT and Aldh1a1−/− mice fed regular chow (B) or HF diet (C), n=4 per group randomly selected from Study 1 and 2. Relative expressions of Rbfox3 (D), Nestin (E), and Synopsin (F) were measured in iAb WAT from WT and Aldh1a1−/− males (triangle, n=5) and females (circle, n=5) by TaqMan RT-PCR (HF, Study 1). Expressions were normalized to Tata box protein (TBP). The levels of peripherin (G) and tyrosine hydroxylase (TH) (H) were analyzed by Western blot from randomly selected tissues of WT (n=4) and Aldh1a1−/− mice (n=4) (HF, Study 1). Protein expression was normalized by levels of housekeeping genes (β-actin). The insert shows representative Western blot. (I) Norepinephrine (NE) level from iAb fat pat homogenates in WT (n=6) and Aldh1a1−/− mice (n=5) was measured using ELISA (HF diet, Study 1). An asterisk indicates a significant difference between WT and Aldh1a1−/− groups (p<0.05). n.s.: not significant, Mann-Whitney U test.
EXPERIMENTAL AND METHODS
Animal studies
Animal studies were approved by the Institutional Animal Care and Use Committee of The Ohio State University (OSU). Data for all studies are summarized in Table S1. The exclusion criteria was the loss of more than 20% of weight in the course of the study; however, no animal was excluded from this study.
Randomization strategy. In studies 1 and 2 mice were randomized into age matching groups with similar percent of male and female mice. For proof-of concept study 3 we used only aged-matched female mice to match sex of implanted immortalized fibroblasts. Mice were randomly selected into control and treatment groups.
1.1. Study 1. WT and Aldh1a1−/− mice on a high-fat (HF) diet
Aldh1a1−/− deficient mice (C57BL/6J background) were developed and provided by G. Duester and colleagues [26] and their metabolic profile was described [20, 24, 27–29]. Matched C57BL/6 (WT) mice were used as control. C57BL/6J (WT) mice were initially purchased from The Jackson Laboratory (Bar Harbor, ME) and then bred at OSU. Mice were fed a regular chow diet (Harlan Teklad, Madison, WI). We used power analysis to determine the sample size of this study based on data from study [30]; however, more animals were enrolled to complete all analysis.
WT and Aldh1a1−/− were fed a high fat diet (HF, 45% kcal from fat, D12451, Research Diet Inc., New Brunswick, NJ) for 180 days (8 month old at dissection) (Numbers, sex, weight are described in Supplementary Table S1). Visceral (intra-abdominal (i-Ab); gonadal) fat was collected for protein, mRNA, and histology. To omit a concern of heterogeneity of adipose tissue, regarding thermogenic/lipogenic adipocyte composition and innervation, we used the whole fat pad for each analysis. For example, we used the whole left fat pad for mRNA analysis and the right for the protein analysis. For histochemistry and NE extraction we used another group of randomly selected animals from the same large study. The number of animals that were randomly selected for specific analysis were smaller than the number of animals in the whole group and are described in figure legends.
Study 2. Comparison of WT and Aldh1a1−/− mice on a regular chow diet
Five-month old WT and Aldh1a1−/− mice (numbers, sex, weight are described in Supplementary Table S1) were fed a regular chow diet (Harlan Teklad, Madison, WI). WAT and EDTA-plasma was collected for analysis and stored at −80°C.
Study 3. WT mice on a high-fat (HF) diet treated with encapsulated WT adipocytes and Aldh1a1−/− adipocytes
The immortalized WT and Aldh1a1−/− were derived from females [28], therefore, we used females for this mechanistic proof-of-concept study. Encapsulation into alginate-poly-L-lysine is described in a detailed video protocol [31]. Eighteen 4-month old WT female mice (C56BL/6J, The Jackson Laboratory, Bar Harbor, ME, breed at OSU) were fed a HF diet for 220 days to induce obesity (N=18; Supplementary Table S1). After that period, mice were randomly assigned into four groups: 1) injected with vehicle (1mL sterile PBS, n=5); 2) acellular ‘empty’ capsules (n=3); 3) encapsulated WT fibroblasts (0.5×106 cells in 1 mL PBS per iAb depot, n=5); and 4) encapsulated Aldh1a1−/− preadipocytes (0.5×106 cells in 1 mL PBS per iAb depot, n=5). Mice were injected with vehicle or encapsulated cells into both iAb depots, and maintained on the same HF diet for additional 80 days. The induction of thermogenesis by encapsulated Aldh1a1−/− under these conditions is described in [28]. Whole iAb fat, which contains all encapsulated and host cells, was collected for protein analysis. Another iAb fat pad was embedded into paraffin for histological examination of innervation.
Metabolic measurements
Metabolic parameters were measured by indirect calorimetry (CLAMS, Columbus Instruments, Columbus, OH) at ambient temperature (22°C) with 12h light/dark cycles. Animals were fed the same HF diet and water was provided ad libitum. Mice were housed individually and allowed to acclimatize in the chambers for 12h. Oxygen consumption, CO2 production, energy expenditure, and locomotor activity were measured for at least 24h. Based on these data, respiratory quotient or exchange ratio (VCO2/VO2) and heat values were calculated by CLAMS.
Norepinephrine (NE) measurements
Norepinephrine was measured in plasma and fat samples using Noradrenaline High Sensitive ELISA (EA633.96, Eagle Biosciences/DLD Diagnostika Hamburg, Germany) according to manufacturer’s instructions. Hemolysed blood samples were excluded as recommended by manufacturer. For analysis the whole fat pad (inguinal, i-Ab, or two brown fat pads) was homogenized using phosphate buffered saline.
Cell culture studies
Stromal vascular fraction (SVF) and immortalized cell line development
SVF was isolated from subcutaneous fat of one-month-old WT and Aldh1a1−/− female mice fed regular chow as described [30]. Fibroblasts (preadipocytes) from SVF were immortalized using the protocol described in [28]. The phenotype of these cells was validated using Affimetrix gene microarray (described below). Full gene expression data set is uploaded as GEO file: ‘QS wild type and Aldh1a1 KO preadipocytes 2015’.
Preparation and stimulation of adult mouse dorsal root ganglion (DRG) neurons
Single cell suspensions from cervical, thoracic, and lumbar DRG neurons were isolated from terminally anesthetized C57BL/6 adult female mice (12–16 weeks old, regular chow, The Jackson Laboratory) [32]. Overall three mice were purchased for DRG isolation and these mice were not a part of other studies. Dissected DRGs were enzymatically digested in a solution of collagenase type 2 (200 U/mL; Sigma-Aldrich C6885, St. Louis, MO) and dispase I (5U/mL; Sigma-Aldrich D4818) on a shaker for 45 min at 37°C [32]. Enzyme solution was aspirated and cells were washed twice in Hank’s Balanced Salt Solution 1× (HBSS; Mediatech Inc., Manassas, VA) before incubating in DNase I type II (5mg/mL; Worthington Biochemical, Lakewood, NJ) for 5 min at room temperature. DRGs were triturated in HBSS with Pasteur pipette until cells were well dissociated, then passed through a Falcon 70μm cell strainer (Corning Inc., Corning, NY) to remove myelin debris, After a 3 min centrifugation at 3,000 rpm to pellet the cells. The neuron-enriched pellet was resuspended in DRG culture medium (DMEM/F12, 1% N2 supplement, and 0.05% Gentamicin) and live cells were counted on a hemocytometer using trypan blue exclusion. Cells were plated at 500 cells/well in a 24-well plate (Corning Inc.) previously coated with poly-D-lysine (25ug/mL; Sigma-Aldrich P6407) and laminin (10 μg/mL; Thermo Fisher Scientific 23017015, Grand Island, NY). Each batch of DRG neurons were incubated at 37°C/5% CO2 in DRG culture medium only, DRG culture medium with NT-3 (1 ng/mL), with NGF (10 ng/mL), with WT secretome, with DRG medium (1:1, v/v), and with Aldh1a1−/− secretome with DRG medium (1:1, v/v) for 24 hours. To assess neurite outgrowth and neuronal morphology, cells were fixed with 4% paraformaldehyde for 25 min [33] and washed in 0.1 M PBS, then incubated in blocking solution (4%BSA/0.3% Tx-100/PBS) for 1 hr at room temperature. Cells were immunostained with a monoclonal neuronal anti-β-tubulin III antibody (Sigma-Aldrich, T8578). Antibody was diluted in blocking solution (1:1000; at 4°C overnight ), then washed and incubated in Alexa Fluor 546 secondary antibody (1:1000; Thermo Fisher Scientific Z25004) for 1 h at RT. Cells were automatically imaged using a Thermo Scientific™ ArrayScan™ XTI Live High Content microscope and analyzed with the Neuronal Profiling algorithm in the HCS Studio™ software (Thermo Fisher Scientific) [34]. Total neurite length, average neurite length, number of branch points, critical value, dendrite maximum, and ramification index were averaged across all neurons detected per well, with 3 wells included per treatment. Three experimental replicates were performed using DRGs from three mice (total: n=9). Dendrite maximum and critical value are parameters determined based on Scholl analysis. These values were automatically generated by the software. Dendrite maximum corresponds to the maximum number of dendrite crossings at a given radius from the cell body, and the critical value describes the radius at which the dendrite max occurs. The ramification index of a neuron is the dendrite max value divided by the number of primary dendrites. Percent of neurons with neurites was calculated as the ratio of the number of neurons with neurite extension to the total number of neurons per well. Significant growth differences were calculated in GraphPad Prism 5.0 (GraphPad Software) using well averages in a one-way ANOVA followed by a Tukey post-hoc analysis. Differences were significant for p<0.05.
Adipocyte differentiation
Murine preadipocyte (3T3-L1, WT, and Aldh1a1−/−) lines were cultured and maintained in standard culture medium (DMEM containing 10% calf serum and 0.1% 50 mg/mL gentamicin). Adipogenesis was induced (Day 0) in confluent preadipocytes using differentiation medium I containing 3-isobutyl-1-methylxanthine (0.5mM), dexamethasone (1μM), insulin (1.7μM), 10% FBS, and 0.1% gentamicin in DMEM. Differentiation medium II containing 10% FBS, insulin (1.7μM), and 0.1% gentamicin in DMEM was replaced every 48 h after adding differentiation medium I.
Gene expression analysis
Affymetrix GeneChip
mRNA was isolated by RNeasy (Qiagen, Valencia, CA). RNA integrity was interrogated using an Agilent 2100 Bioanalyzer (Agilent Technologies). A 100 ng aliquot of total RNA was linearly amplified. Then, 5.5μg of cDNA was labeled and fragmented using the GeneChip WT PLUS reagent kit (Affymetrix, Santa Clara, CA) following the manufacturer’s instructions. Labeled cDNA targets were hybridized to Affymetrix GeneChip Mouse Gene ST 2.0 arrays for 16 h at 45 °C rotating at 60 rpm. The arrays were washed and stained using a Fluidics Station 450 and scanned using a GeneChip Scanner 3000. Signal intensities were quantified by Affymetrix Expression Console version 1.3.1. Background correction and quantile normalization were performed to adjust for technical bias, and probe-set expression levels were calculated by the RMA method. After filtering above noise cutoff, there are 9,528 probe-sets that were tested by linear model. A variance smoothing method with fully moderated t-statistic was employed for this study and was adjusted by controlling the mean number of false positives. With a combined cutoff of 2-fold change and p-value of 0.0001 (controlling 1 false positive over all probe-sets), we declared 500 probe-sets as differential gene expression between Aldh1a1−/− and WT preadipocytes. GEO file: ‘QS wild type and Aldh1a1 KO preadipocytes 2015’.
NanoString nCounter gene expression assay
NanoString’s nCounter analysis (NanoString Technologies) system performed direct detection of target molecules from a single sample using color-coded molecular barcodes, giving a digital quantification of the number of target molecules as described before [35]). A custom panel containing axon guidance molecules was designed and used for simultaneous quantification of 32 axon guidance genes and 5 housekeeping genes. All data were normalized to 5 housekeeping genes quantified in the same samples. Total mRNA (100ng in 5ul) was hybridized overnight with nCounter Reporter (20μl) probes in hybridization buffer and in an excess of nCounter Capture probes (5μL) at 65°C for 16–20h. The hybridization mixture containing target/probe complexes was allowed to bind to magnetic beads containing complementary sequences on the Capture Probe. After each target found a probe pair, excess probes were washed followed by sequential binding to sequences on the Reporter Probe. Biotinylated capture probe-bound samples were immobilized and recovered on a streptavidin-coated cartridge. The abundance of specific target molecules was then quantified using the nCounter Digital Analyzer. Individual fluorescent barcodes and target molecules present in each sample were recorded with a CCD camera by performing a high-density scan (600 fields of view). Images were processed internally into a digital format and were normalized using the NanoString nSolver software analysis tool. Counts were normalized for all target RNAs in all samples based on the positive control RNA to account for differences in hybridization efficiency and post-hybridization processing, including purification and immobilization of complexes. The average was normalized by background counts for each sample obtained from the average of the eight negative control counts. Six internal reference housekeeping genes: Gapdh, Gusb, Hprt1, Pgk1, and Tubb were analyzed. Subsequently, a normalization of mRNA content was performed based on these genes, using nSolver Software
Semi-quantitative mRNA analysis
mRNA was isolated from WAT or adipocyte cultures according to the manufacturer’s instructions (Qiagen; Valencia, CA). cDNA was prepared from purified mRNA and analyzed using a 7900HT Fast Real-Time PCR System, TaqMan fluorogenic detection system, and validated primers (Applied Biosystems; Foster City, CA). Comparative real time PCR was performed in triplicate, including no-template controls. mRNA expression of genes of interest was normalized by Tata box protein (TBP) or18S expression level using the comparative cycle threshold (Ct) method.
Protein analysis
Immunohistochemistry
Fat pads were embedded into paraffin for immunohistochemical analysis. Sections were stained with hematoxylin and eosin (H&E) using a modified hematoxylin procedure followed by dehydration in graded alcohol or with peripherin (Abcam, ab4666, Cambridge, MA) and tyrosine hydroxylase polyclonal rabbit antibodies (Abcam, ab41528, Cambridge, MA) at 1:1000 dilution. Images were obtained using Olympus M081 IX50 and Pixera Viewfinder 3.0 software.
Western blot
Cell/tissue protein lysates were normalized by protein content (BCA, Thermo Fisher Scientific). Medium was collected from cells, which were plated at similar numbers. Protein lysate or medium were separated on 10% acrylamide gel under reducing conditions. After transfer to a polyvinylidene fluoride membrane (Immobilon-P; Millipore), proteins were analyzed using an Odyssey Infrared Imaging System (LI-COR Biosciences). EFNA5 (Abcam, ab70114, Cambridge, MA), peripherin, and tyrosine hydroxylase polyclonal rabbit antibodies (same as in immunohistochemistry) were used at 1:1000 dilution.
Enzyme-linked immunosorbent assay (ELISA)
Plasma samples were collected from WT and Aldh1a1−/− (A1KO) mice in Study 1–3. Adipose tissues were homogenized in radio immunoprecipitation assay (RIPA) buffer and used at same protein concentration (50μg/mL). Medium was collected from differentiated and non-differentiated 3T3-L1. Absorbance (450 nm) was measured using a Synergy H1 Hybrid Multi-Mode Microplate Reader. NGF ELISA was purchased from Abnova (KA0400, Walnut, CA) and used to measure media from WT and Aldh1a1−/− adipocytes and plasma from WT and Aldh1a1−/− mice.
Statistical analysis
Blinding statement
The mice treatment and collection of metabolic data and analysis of mRNA samples and immunohistochemistry was performed by different groups on coded samples. Human samples were obtained at Mayo clinic, and animal studies were performed by Ms. Shen and Yasmeen, who were responsible for the sample coding. The coded animals were placed in metabolic cages facility supervised by the Department of Physiology and data were collected and digitalized. mRNA and gene array was performed on coded samples at Nucleic acid facility and Dr. Fadda performed basic normalization on coded samples. Statistical analysis was performed by Dr Yu at the statistical department, who was provided with the decoding information. Dr. Flechtner performed immunohistochemistry on coded samples. Ms. Marbourg performed DRG experiments and axon outgrowth for each condition without knowledge to treatment. Unbiased analyses of axon growth were conducted using software (HCS Studio™ software) associated with an automated high-content image analysis system.
All data are reported either as mean±standard deviation (s,d.) or mean± standard error (s.e.m). Number of samples is indicated in figure legends and Supplementary Table S1. Normal distribution was tested using Shapiro-Wilk test. Group comparisons were assessed using Mann Whitney U test, and/or ANOVA models or Kolmogorov-Smirnov test. All tests were two-sided. P<0.05 was considered to be statistically significant and is presented as asterisk (*). Trends were examined using Pearson correlation analysis tests.
Supporting Information Available
Supplementary Table S1, and Supplementary Figure S1 are provided.
RESULTS
Aldh1a1 in WAT creates a permissive environment for neural innervation
We investigated the cumulative impact of Aldh1a1 deficiency in peripheral innervation in mice adipose tissue. NGF was the first neurotrophic factor, which was described in adipocytes to be associated with obesity and vitamin A [36–38]. NGF levels in the circulation were similar in WT and Aldh1a1−/− mice fed a regular (Figure 1B, Study 2) and a high fat chow (HF, Figure 1C, Study 1). Next, we compared expression of neuronal markers in WT and Aldh1a1−/− male and female mice developing different WAT mass on a HF diet. Expression of Rbfox3 (Figure 1D), a marker of the mature neurons, was similar in WAT of WT and Aldh1a1−/− male and female mice on a HF diet. The expression of the neuronal precursor markers nestin (Figure 1E) and synapsin (Figure 1F) was significantly reduced in Aldh1a1−/− compared to WT mice on a HF diet. These data suggest diminished de novo neurogenesis from the stromal cells or preadipocytes. This was expected due to diminished RA production in WAT in Aldh1a1−/− vs. WT adipocytes [20] and in the circulation [39]. Sprouting and growth of axons could increase innervation [11]. We observed higher protein expression in peripherin and tyrosine hydroxylase (TH), markers of peripheral and sympathetic neurons, respectively in Aldh1a1−/− vs. WT WAT (Figure 1G, H). The increased innervation was associated with significantly higher levels of norepinephrine (NE) in WAT of Aldh1a1−/− compared to WT mice (Figure 1I). We hypothesized that Aldh1a1−/− preadipocytes and/or adipocytes release signals promoting innervation by growing axons.
Aldh1a1−/− adipocytes stimulate growth in dorsal root ganglion (DRG) neurons in vitro
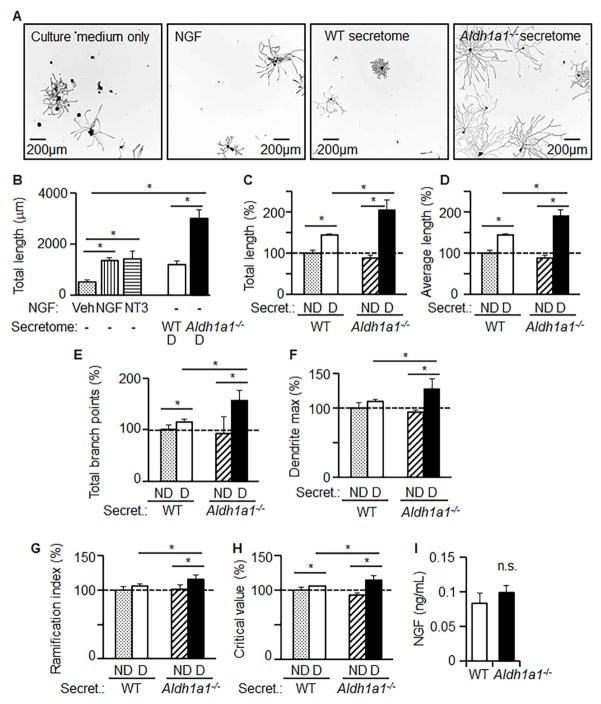
To prove that molecules secreted from Aldh1a1−/− preadipocytes can directly influence neurons, we stimulated neurons with a medium from differentiated WT and Aldh1a1−/− adipocytes. For all experiments, we isolated an equal number of DRG neurons, 500 per well. Axonal growth in media from Aldh1a1−/− adipocytes was significantly more extensive than that seen in the presence of the classic inducers, NGF (Figure 2A, B, larger numbers are shown in Supplementary Figure S1). Stimulation of primary murine DRG neurons with the medium obtained from differentiated Aldh1a1−/− adipocytes led to a marked induction of axonal growth. We quantified axon growth by total length (Figure 2C), average length (Figure 2D), total branching points (Figure 2E), dendrite max (Figure 2F), as well as ramification index (Figure 2G) and critical values calculated from these parameters (Figure 2H). All parameters showed that media from differentiated Aldh1a1−/− adipocytes vs. WT adipocytes markedly increased axon growth. Endogenous levels of NGF were present in low and equal amounts in the media from WT adipocytes and Aldh1a1−/− adipocytes (0.1ng/mL compared to 10ng/mL of NGF used for positive control) (Figure 2I). Thus, molecules secreted from Aldh1a1−/− adipocytes are neurotrophic and promote robust axon growth.
Figure 2. Secretome of Aldh1a1−/− thermocytes promotes axon growth in DRG neurons in vitro.
DRG neurons (500 neurons per well) were cultured in DRG culture medium only, or DRG culture medium with NGF (10ng/mL), or DRG culture medium with WT secretome (1/1, v/v), or DRG culture medium with Aldh1a1−/ − secretome (1/1, v/v) for 24 hours. WT and Aldh1a1−/ − adipocytes were differentiated for 5 days. The secretome is the media collected from these fully-differentiated cells after 24h of incubation. Neurite outgrowth parameters were assayed using a Thermo Scientific™ ArrayScan™ XTI Live High Content microscope and analyzed with the Neuronal Profiling Algorithm. Nine independent experiments were performed using DRG from three mice. Each DRG batch was analyzed in triplicate. Asterisks indicate P<0.05 between different groups; Mann-Whitney U test. Representative images (A) and quantification of neurite length (B) were taken in neurons 24 hours after stimulation. (C–F) Axon guidance effects of secretomes from non-differentiated WT (ND, dotted bars) and Aldh1a1−/ − (ND, upward hatched bars) preadipocytes, and differentiated (D, 5 days) WT (white bars) and Aldh1a1−/ − (black bars) adipocytes on DRG neurons. Total length (C), average length (D), total branch points (E), dendrite maximum length (F), ramification index (G), as well as critical value (H) were measured in DRG neurons and analyzed by Neuronal Profiling Algorithm. (I) NGF levels in media collected from differentiated WT and Aldh1a1−/ − adipocytes (n=4 per group) were analyzed by ELISA. Data show mean ± SD obtained with one DRG batch. Asterisks indicate P<0.05 between different groups; Mann-Whitney U test.
RA suppressed the positive regulation of axon growth by Aldh1a1−/− secretome
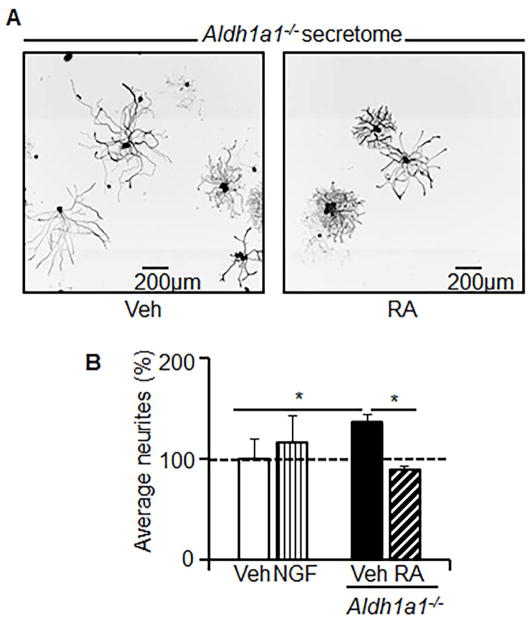
To test the role of RA in regulation of axon-guiding mediators in Aldh1a1−/− adipocytes, we differentiated Aldh1a1−/− preadipocytes in the presence or absence of RA. Then we cultured naïve primary DRG neurons in this medium. The RA-conditioned Aldh1a1−/− secretome inhibited the growth of DRG axons relative to the neurotrophic media from Aldh1a1−/− adipocytes (Figure 3A, B). Thus, intracrine regulation of RA production from Rald by ALDH1A1 may be a mechanism by which adipocytes control innervation.
Figure 3. The axon-growth supportive milieu of Aldh1a1−/− secretome is RA-dependent.
DRG neurons were cultured in DRG culture medium with the secretome from Aldh1a1−/− adipocytes (1/1, v/v) that were treated with or without RA (100nM). RA was added to Aldh1a1−/− differentiation medium (Day 0, 2, 5). To prepare this secretome, medium was collected for the last 24 hours. (A) Representative images were selected from 3 independent experiments. (B) Mean neurite growth indices were measured by XTI microscopy in DRG neurons in DRG culture medium containing Veh (vehicle, white bar), NGF (vertical lined bar), or Aldh1a1−/− secretome treated without (black bar) or with RA (hatched bar). An asterisk indicates P<0.05; Mann-Whitney U test.
Aldh1a1 regulates genes involved in axon guidance
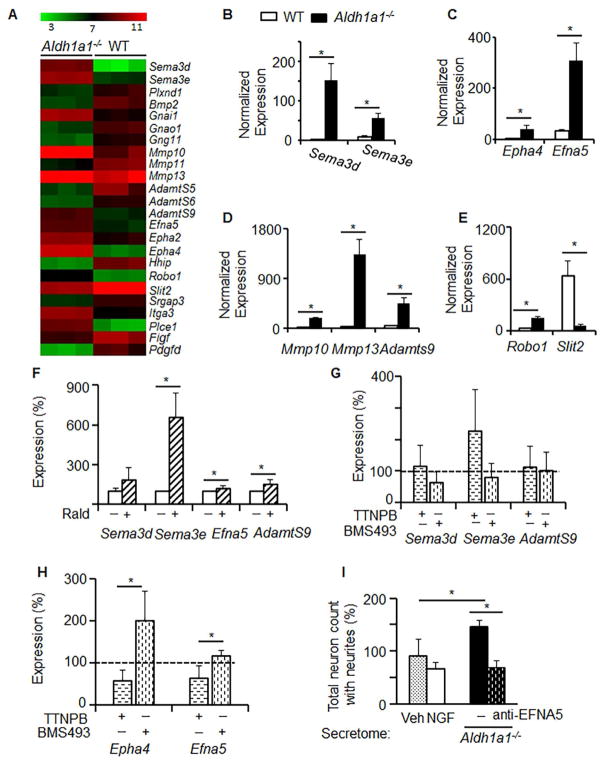
We compared genome of WT and Aldh1a1−/− preadipocytes using gene microarray (Figure 4A). Pathway analysis revealed that the primary pathway altered in Aldh1a1−/− vs. WT adipocytes was the axon guidance (Figure 4A). Axon guidance pathway includes axon guidance molecules Efna5, Sema3d, Sema3e, their receptors and co-regulatory molecules Epha4, Slit1, Robo1, Hhip. [40, 41] Axon growth is dependent on proteolysis that enables remodeling of matrix proteins and cleavage-dependent activation and secretion of ephrin ligands [42, 43]. Within the Aldh1a1−/− -axon guidance pathway, there was significantly increased expression of mRNA encoding the proteases AdamtS9, matrix metallopeptidase 10 (Mmp10), and Mmp13 in Aldh1a1−/− compared to WT adipocytes. The changes in expression of all these genes were validated using the quantitative nanostring approach (Figure 4B–E).
Figure 4. Aldh1a1−/− adipocytes express axon guidance molecules regulated by RAR.
(A) Expression heat map reveals differences in expression of axon guidance cluster of genes between WT and Aldh1a1−/− preadipocytes (n=3 per group). Transcript analysis was performed by Affymetrix microarray. The axon guidance cluster was identified by Ingenuity Pathway Analysis. (B–E) WT (white bars) and Aldh1a1−/− (black bars) preadipocytes (n=3) were differentiated for 4 days. Expressions of Sema3d and Sema3e (B); Epha4 and Efna5 (C); Mmp10, Mmp13, and Adamts9 (D); Robo1 and Slit2 (E) were measured in purified mRNA samples using a customized NanoString mouse panel including markers for adipogenesis, thermogenesis, and axon guidance genes. Data represent mean±SD. An asterisk indicates p<0.05; Mann Whitney U test. (F–H) 3T3-L1 adipocytes were cultured in differentiation medium for 4 days. At day 5, medium was replaced by UV-treated 1% FBS-containing DMEM with and without Rald (30nM) (F), or with and without TTNPB (50nM) or BMS493 (100nM) (G–H). 24 hrs post stimulation, cells were harvested for mRNA. Gene expression was measured by the NanoString mouse innervation panel. Data (mean±SD, n=3) were normalized to the levels found in non-treated control (100%, dashed line). An asterisk indicates p<0.05; Mann-Whitney U test. (I) Neuron growth indexes were obtained by XTI microscopy in DRG neurons cultured in DRG culture medium with or without NGF or in the Aldh1a1−/− secretome (1/1, v/v) with and without blocking antibody against EFNA5 (1μg/mL) for 24 hours.
To elucidate if Aldh1a1 could be involved in the cumulative regulation of genes responsible for axon guidance, we stimulated 3T3-L1 adipocytes with natural vitamin A metabolites controlled by this enzyme: 1) RA and 2) Rald, a RA precursor present in Aldh1a1−/− adipocytes [27]. We also compared these responses to synthetic RAR agonist TTNPB and antagonist BMS493. We have found that Rald significantly induced the expression of Sema3e, AdamtS9, and Efna5 in 3T3-L1 adipocytes (Figure 4F). However, the ability of Rald to induce expression of Sema3e and AdamtS9 occurs independently of RAR agonists or antagonists (Figure 4G). In contrast, expression of Epha4 and Efna5 was increased in the presence of the RAR inhibitor, BMS493, and suppressed by the RAR ligand, TTNPB (Figure 4H), suggesting direct regulation by RAR. In a loss-of-function experiment, the addition of an anti-EFNA5 antibody prevented outgrowing of neurites and decreased their maximum length following stimulation by Aldh1a1−/− media (Figure 4I). Other parameters, such as total branch point, were not influenced by this treatment. These data suggest that complete Aldh1a1−/− secretome is required to achieve full axonal growth efficiency, although EFNA5 contributes to the axon growth-promoting activity of Aldh1a1−/− adipocytes.
Encapsulated Aldh1a1−/− adipocytes induce innervation in WAT of WT obese mice
To study if Aldh1a1−/− adipocytes could be used to stimulate innervation in vivo, we used a previously established model for testing encapsulated cells for metabolic disease treatment [28, 31]. Semipermeable poly-L-lysine membrane allows encapsulated cells to exchange low molecular weight molecules (<36kD) [28]. Here we used same model to study paracrine effects of axon guidance molecules, because Efna5 gene encodes 26kD protein.
We prepared equal concentrations of empty acellular capsules, encapsulated WT, as well as Aldh1a1−/− adipocytes as previously described [31]. Host obese WT mice were treated using encapsulated WT adipocytes (Control), Aldh1a1−/− adipocytes, or acellular capsules (acellular control). Capsulates were injected into WAT of obese WT host mice Study 3. After 80 days post-treatment WAT were isolated and used for analysis of innervation. Encapsulated Aldh1a1−/− adipocytes, but not WT adipocytes, stimulated the development of peripherin-positive axons around the capsules (Figure 5A). Peripherin expression in WAT injected with encapsulated Aldh1a1−/− adipocytes was significantly higher than in WAT injected with empty capsules (Figure 5B). Tyrosine hydroxylase (TH)-positive sympathetic axons were also present around Aldh1a1−/− capsules, but neither proximal or distal to encapsulated WT adipocytes (Figure 5C). No detectable nerves were found in adipose tissue in non-treated obese mice or mice treated with acellular capsules. Mice treated with encapsulated Aldh1a1−/− adipocytes had increased metabolic rate during the active (dark) period (Figure 6A). This change was not due to differences in food consumption (Figure 6B), as mice injected with encapsulated WT and Aldh1a1−/− cells had similar food intake. The development of sympathetic innervation (Figure 6C) was associated with the formation of thermogenic UCP-1 positive adipocytes clusters in obese WT mice treated with encapsulated Aldh1a1−/− adipocytes described previously [28]. No such effect was seen in control groups. Thus, paracrine effects from a subset of encapsulated Aldh1a1−/− adipocytes promote functional innervation of WAT in vivo.
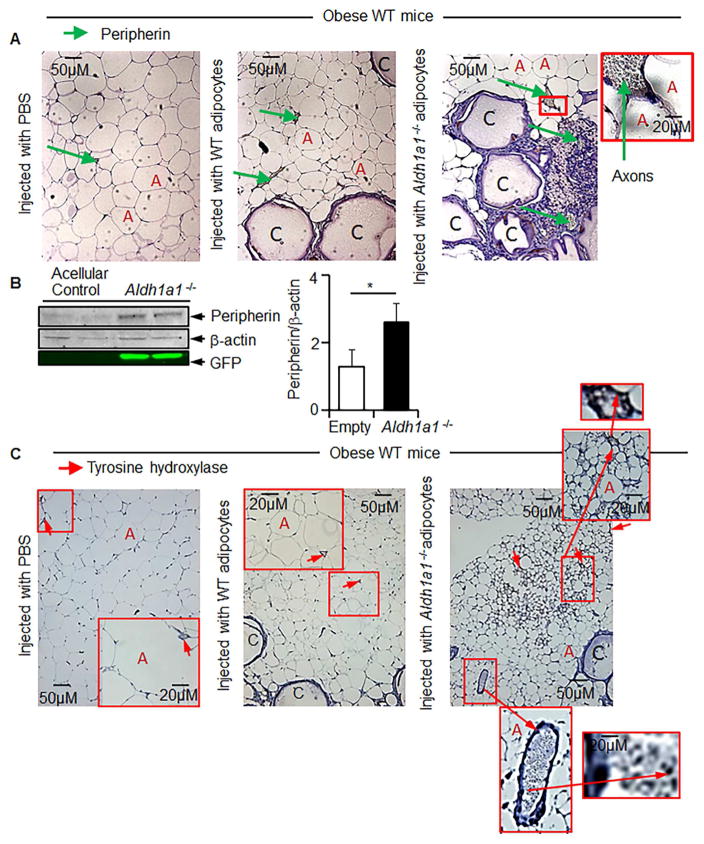
Figure 5. Secretome of grafted encapsulated Aldh1a1−/−adipocytes promotes innervation in WAT in WT obese mice.
Obese WT female mice (n=18) on a HF diet were treated with acellular (n=3) microcapsules or micro capsules containing WT (n=5) or Aldh1a1−/− adipocytes (n=5) or mice remained untreated (n=5) (Study 3). (A) Immunohistochemical analysis of peripherin protein levels in axons in iAb WAT of the animal groups in Study 3. (A) Representative images of peripherin (brown) immunoreactive areas of nerves in paraffin-embedded iAb fat from mice injected with vehicle and encapsulated WT and Aldh1a1−/− cells at 20× magnification. Green arrows indicate examples of peripherin-positive axons of nerves at 40× magnification. ‘C’ indicates empty core of microcapsules because encapsulated cells are attached at the inner surface of capsules. The ‘A’ letters show examples of adipocytes. (B) Peripherin was analyzed by Western blot in whole homogenized iAb WAT pads from mice in Study 3. Bars show peripherin levels from the same tissues normalized to β-actin levels (n=4). An asterisk indicates a significant difference between empty and Aldh1a1−/− groups (p<0.05; Mann-Whitney U test). (C) Immunohistochemical analysis of tyrosine hydroxylase (TH, brown) protein levels in axons of the animal groups in Study 3. Note that TH-positive areas are found within nerves (40×) (red arrow) or in areas containing numerous multilocular adipocytes.
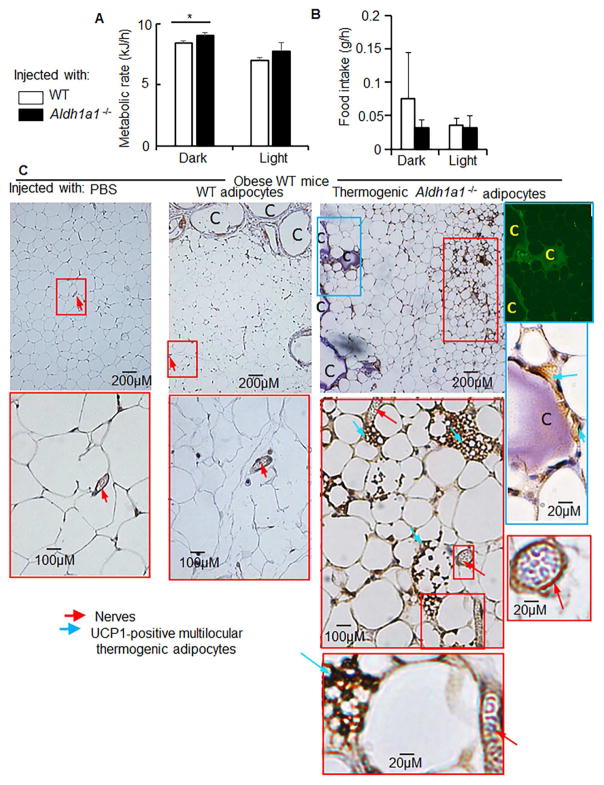
Figure 6. Secretome of grafted encapsulated Aldh1a1−/− adipocytes regulated metabolism and thermogenic properties of WAT in WT obese mice.
Metabolic rate (A) and food intake (B) in the same obese WT mice treated with encapsulated WT adipocytes (white bar) or Aldh1a1−/− adipocytes. Asterisk indicates statistically significant differences in metabolic rate during the dark period between treated groups (Wilcoxon-Mann-Whitney Rank test, p<0.05, n=4). Food intake was not significantly different between groups.
(C) Immunohistochemical analysis of UCP1 levels (marker of thermogenesis) in paraffin-embedded iAb fat from mice injected with vehicle, encapsulated WT, and encapsulated Aldh1a1−/− cells at 20× magnification. Inserted figure shows the example of UCP-1 positive multilicular adipocytes at 40× magnification. These UCP-1 positive thermogenic multilicular adipocyte areas (blue arrows) were localized in proximity of nerves (red arrows). Right insert: Aldh1a1−/− adipocytes inside capsules (indicated by C) also expressed UCP-1.
DISCUSSION
Our studies revealed a specific paracrine role of Aldh1a1−/− adipocytes in the regulation of axon-guidance pathway molecules that support the induction of sympathetic innervation. Moreover, encapsulation of Aldh1a1−/− adipocytes in alginate poly-L-lysine capsules is a viable strategy for boosting sympathetic innervation of WAT over a long period. Indeed, conditioned medium from Aldh1a1−/− adipocytes has potent growth-promoting effects and encapsulation of these adipocytes with subsequent transplantation into WAT maintains their growth-promoting potential in vivo. Encapsulation technology has been previously developed for the implantation of pancreatic beta islet for insulin production [44, 45]. Here we show that a subset of encapsulated cells with the engineered paracrine mechanism can alter expression of multiple genes and orchestrate a complex innervation process. This technology holds the potential to stimulate axonal growth in translational settings for the treatment of metabolic neuropathies and, potentially, injuries and degenerative disease of the CNS.
Studies in animal models of obesity, as well as epidemiologic and clinical evidence, suggest a pathophysiologic relationship between neuropathy and metabolic syndrome [46]. Lipolysis and thermogenesis induction depend on a sensory-sympathetic circuit [10, 11]. Researchers have shown that lipid-storing adipocytes in WAT contribute to the production of axon guiding molecules associated with obesity [38, 47]. Accordingly, the levels of these molecules, such as NGF [37, 38] and SEMA3A [47] are increased in obese patients. We provide evidence of another pathway in which Aldh1a1−/− adipocytes produce distinct guidance cues for sensory axons and establish the inflow from WAT to the brain for the induction of sensory-sympathetic circuit and thermogenesis (Figures 5 and 6).
The key role of ALDH1A1 is in the production of RA from the precursor Rald with downstream activation of RAR in adipocytes [20]. We have shown that Rald can induce expression of Efna5/Epha4 genes. Conversely, conversion of Rald to RA activated RAR and reduced EFNA5 expression and secretion, Therefore, Efna5/Epha4 appear to be the primary target of vitamin A pathway that is responsible for innervation. It is possible that activated RAR also regulate other genes, such as Sema3e. Even though multiple genes are involved in axonal guidance, RAR exerts the overall control over peripheral innervation. Inhibition of RAR decreased neurogenesis, instead, increased EFNA5 expression and secretion stimulated sensory-sympathetic innervation and adaptive thermogenesis. These responses place ALDH1A1 in a central regulatory position switching between lipogenic and thermogenic innervation in WAT.
Several questions remain unanswered from our study. What types of cells are suitable for encapsulation technology and for guidance of specific neurons subtypes? Many empirical studies have indicated that stem cells in adipose tissue can improve recovery from spinal cord injuries [48]. Other researchers have noted that implants of thermogenic beige and brown adipocyte become innervated, without explaining the mechanism of this phenomenon [49]. It is unclear if these cells play a paracrine role in innervations or differentiate precursor cells into neurons. Importantly, small populations of adipocytes in WAT can produce cues to promote de novo innervation and can be used for encapsulation technology. More research is needed to clarify the composition of molecules associated with axon guidance, which could include microRNA and/or metabolites. We did not investigate these molecules in current study. Recent studies showed that AdamtS9 is a gene associated with sexual dimorphism in fat distribution [50] as well as susceptibility to type 2 diabetes in adults [51] and children [52]. Further studies are needed to elucidate if AdamtS9 supports the EFNA5/EPHA4 pathway of axon growth and if it contributes to the role of EFNA5 in obesity. The primary goal of this study was to prove that Aldh1a1−/− adipocytes use a paracrine mechanism to induce axon growth in WAT. In vitro, the secretome of Aldh1a1−/− adipocytes robustly induced neurite outgrowth in DRG neurons and its activity exceeded effects of the classic inducers, NGF and NT-3. In vivo, only encapsulated Aldh1a1−/− adipocytes, stimulated innervation in WAT of obese WT host mice. The nerves adjacent to encapsulated Aldh1a1−/− adipocytes were peripherin- and TH-positive, indicating sympathetic innervation. This innervation was associated with increased NE secretion and converted adipocytes of obese WT animals into thermogenic UCP1-positive multilocular adipocytes. This conversion increased the metabolic rate of treated animals. The remodeling of host WAT occurred distally from capsules by means of the outgrowth of host TH-positive sympathetic neurons. Therefore, paracrine mediators from Aldh1a1−/− adipocytes, acting via the sympathetic nerves system, have a higher capacity for thermogenic remodeling than stimulation of an intracellular thermogenic machinery in beige or brown adipocytes. This autonomous mechanism induced by encapsulated engineered cells offers an opportunity for treatment of obesity in a fat depot-specific manner.
Supplementary Material
Acknowledgments
We would like to thank Alessandro Brunetti, Jennifer Wall, and Jack Leung (The Ohio State University) for their editorial help. This research was supported by the Ray W. Poppleton Endowment (P.G.P.), and NSF Center funding (EEC-0914790). The project was supported by NIH grants R21OD017244 (O.Z.), R01NS047175 (P.G.P.), R00 HL121284-01 (F.A), the National Center for Research Resources UL1RR025755, UL1TR001070, and NCI P30CA16058 (OSUCCC), and the NIH Roadmap for Medical Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. This research was supported by the SEED Grant from College of Education and Human Ecology, Accelerator Grant from Office for International Affairs, and Center for Advanced Functional Foods Research and Entrepreneurship at OSU (O.Z.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Advancing Translational Sciences. All authors have read the journal’s authorship agreement and the manuscript has been reviewed by and approved by all named authors.
Abbreviations
- A1KO
Aldehyde dehydrogenase 1 A1 deficient mice (Aldh1a1−/−)
- Aldh1a1
Aldehyde dehydrogenase family 1 member A1
- ANOVA
Analysis of variance
- CNS
Central nervous system
- DRG
Dorsal root ganglia
- EFNA5
Ephrin A5 ligand
- ELISA
Enzyme-linked immunosorbent assay
- EPHA4
Ephrin A4 receptor
- iAb
intra-abdominal or visceral fat
- HF
High-fat
- Mmp
Matrix metallopeptidase
- NE
Norepinephrine
- NGF
Nerve growth factor
- PBS
Phosphate buffered solution
- PNS
Peripheral nervous system
- RA
Retinoic acid
- Rald
Retinaldehyde or retinal
- RAR
Retinoic acid receptor
- rEFNA5
Recombinant ephrin A5
- RER
Respiratory exchange ratio
- ROL
Retinol
- RT
Room temperature
- Sema3e
Semaphorin 3e
- Sema3d
Semaphorin 3d
- SNS
Sympathetic nervous system
- TBP
Tata box protein
- TH
Tyrosine hydroxylase
- TTNPB
Arotinoid acid or 4-[(E)-2-(5,6,7,8-Tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl)-1-propenyl]benzoic acid (high affinity RAR agonist)
- UCP1
Uncoupling protein 1
- Veh
Vehicle
- WAT
White adipose tissue
- WT
Wild type
Footnotes
The authors declare that they have no conflict of interests.
CONFLICT OF INTERESTS
The authors declare that they have no conflicts of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Inskip J, Plunet W, Ramer L, et al. Cardiometabolic risk factors in experimental spinal cord injury. Journal of neurotrauma. 2010;27(1):275–85. doi: 10.1089/neu.2009.1064. [DOI] [PubMed] [Google Scholar]
- 2.Edwards LA, Bugaresti JM, Buchholz AC. Visceral adipose tissue and the ratio of visceral to subcutaneous adipose tissue are greater in adults with than in those without spinal cord injury, despite matching waist circumferences. Am J Clin Nutr. 2008;87(3):600–7. doi: 10.1093/ajcn/87.3.600. doi. [DOI] [PubMed] [Google Scholar]
- 3.Hetz C, Saxena S. ER stress and the unfolded protein response in neurodegeneration. Nature reviews Neurology. 2017 doi: 10.1038/nrneurol.2017.99. [DOI] [PubMed] [Google Scholar]
- 4.Rojas-Gutierrez E, Munoz-Arenas G, Trevino S, et al. Alzheimer’s disease and metabolic syndrome: A link from oxidative stress and inflammation to neurodegeneration. Synapse. 2017 doi: 10.1002/syn.21990. [DOI] [PubMed] [Google Scholar]
- 5.Andres RC, Helena BC, Juliana PP, et al. Diabetes-related neurological implications and pharmacogenomics. Current pharmaceutical design. 2017 doi: 10.2174/1381612823666170317165350. [DOI] [PubMed] [Google Scholar]
- 6.De La Monte SM. Metabolic derangements mediate cognitive impairment and Alzheimer’s disease: role of peripheral insulin-resistance diseases. Panminerva medica. 2012;54(3):171–8. doi. [PMC free article] [PubMed] [Google Scholar]
- 7.Lu M, Hu G. Targeting metabolic inflammation in Parkinson’s disease: implications for prospective therapeutic strategies. Clinical and experimental pharmacology & physiology. 2012;39(6):577–85. doi: 10.1111/j.1440-1681.2011.05650.x. [DOI] [PubMed] [Google Scholar]
- 8.Adams RH, Eichmann A. Axon guidance molecules in vascular patterning. Cold Spring Harbor perspectives in biology. 2010;2(5):a001875. doi: 10.1101/cshperspect.a001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartness TJ, Song CK. Thematic review series: adipocyte biology. Sympathetic and sensory innervation of white adipose tissue. Journal of lipid research. 2007;48(8):1655–72. doi: 10.1194/jlr.R700006-JLR200. [DOI] [PubMed] [Google Scholar]
- 10.Ryu V, Bartness TJ. Short and long sympathetic-sensory feedback loops in white fat. American journal of physiology Regulatory, integrative and comparative physiology. 2014;306(12):R886–900. doi: 10.1152/ajpregu.00060.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartness TJ, Ryu V. Neural control of white, beige and brown adipocytes. International journal of obesity supplements. 2015;5(Suppl 1):S35–9. doi: 10.1038/ijosup.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell. 2014;156(1–2):20–44. doi: 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giger RJ, Hollis ER, 2nd, Tuszynski MH. Guidance molecules in axon regeneration. Cold Spring Harbor perspectives in biology. 2010;2(7):a001867. doi: 10.1101/cshperspect.a001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma CH, Omura T, Cobos EJ, et al. Accelerating axonal growth promotes motor recovery after peripheral nerve injury in mice. The Journal of clinical investigation. 2011;121(11):4332–47. doi: 10.1172/JCI58675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scheib J, Hoke A. Advances in peripheral nerve regeneration. Nature reviews Neurology. 2013;9(12):668–76. doi: 10.1038/nrneurol.2013.227. [DOI] [PubMed] [Google Scholar]
- 16.Gaudet AD, Popovich PG, Ramer MS. Wallerian degeneration: gaining perspective on inflammatory events after peripheral nerve injury. Journal of neuroinflammation. 2011;8:110. doi: 10.1186/1742-2094-8-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santos D, Giudetti G, Micera S, et al. Focal release of neurotrophic factors by biodegradable microspheres enhance motor and sensory axonal regeneration in vitro and in vivo. Brain research. 2016;1636:93–106. doi: 10.1016/j.brainres.2016.01.051. [DOI] [PubMed] [Google Scholar]
- 18.Eyjolfsdottir H, Eriksdotter M, Linderoth B, et al. Targeted delivery of nerve growth factor to the cholinergic basal forebrain of Alzheimer’s disease patients: application of a second-generation encapsulated cell biodelivery device. Alzheimer’s research & therapy. 2016;8(1):30. doi: 10.1186/s13195-016-0195-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sorrell JM. Development of arteries in embryonic chick bone marrow with special reference to the appearance of periarterial granulopoietic sheaths. The Anatomical record. 1988;221(3):730–6. doi: 10.1002/ar.1092210308. [DOI] [PubMed] [Google Scholar]
- 20.Reichert B, Yasmeen R, Jeyakumar SM, et al. Concerted action of aldehyde dehydrogenases influences depot-specific fat formation. Mol Endocrinol. 2011;25(5):799–809. doi: 10.1210/me.2010-0465. me.2010-0465 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yasmeen R, Jeyakumar SM, Reichert B, et al. The contribution of vitamin A to autocrine regulation of fat depots. Biochimica et biophysica acta. 2012;1821(1):190–7. doi: 10.1016/j.bbalip.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu S, Levi L, Siegel R, et al. Retinoic acid induces neurogenesis by activating both retinoic acid receptors (RARs) and peroxisome proliferator-activated receptor beta/delta (PPARbeta/delta) The Journal of biological chemistry. 2012;287(50):42195–205. doi: 10.1074/jbc.M112.410381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonet ML, Ribot J, Palou A. Lipid metabolism in mammalian tissues and its control by retinoic acid. Biochimica et biophysica acta. 2012;1821(1):177–89. doi: 10.1016/j.bbalip.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Yasmeen R, Reichert B, Deiuliis J, et al. Autocrine Function of Aldehyde Dehydrogenase 1 as a Determinant of Diet- and Sex-Specific Differences in Visceral Adiposity. Diabetes. 2012 doi: 10.2337/db11-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiefer FW, Vernochet C, O’Brien P, et al. Retinaldehyde dehydrogenase 1 regulates a thermogenic program in white adipose tissue. Nature medicine. 2012;18(6):918–25. doi: 10.1038/nm.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan X, Molotkov A, Manabe S, et al. Targeted disruption of Aldh1a1 (Raldh1) provides evidence for a complex mechanism of retinoic acid synthesis in the developing retina. Molecular and cellular biology. 2003;23(13):4637–48. doi: 10.1128/MCB.23.13.4637-4648.2003. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ziouzenkova O, Orasanu G, Sharlach M, et al. Retinaldehyde represses adipogenesis and diet-induced obesity. Nature medicine. 2007;13(6):695–702. doi: 10.1038/nm1587. nm1587 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang F, Zhang X, Maiseyeu A, et al. The prolonged survival of fibroblasts with forced lipid catabolism in visceral fat following encapsulation in alginate-poly-L-lysine. Biomaterials. 2012;33(22):5638–49. doi: 10.1016/j.biomaterials.2012.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gushchna L, Yasmeen R, Ziouzenkova O. Moderate vitamin A supplementation in obese mice regulates tissue factor and cytokine production in a sex-specific manner. Archives of Biochemistry and Biophysics. 2013;539:239–47. doi: 10.1016/j.abb.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yasmeen R, Reichert B, Deiuliis J, et al. Autocrine function of aldehyde dehydrogenase 1 as a determinant of diet- and sex-specific differences in visceral adiposity. Diabetes. 2013;62(1):124–36. doi: 10.2337/db11-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu L, Shen Q, Mao Z, et al. Encapsulation Thermogenic Preadipocytes for Transplantation into Adipose Tissue Depots. Journal of visualized experiments : JoVE. 2015;(100):e52806. doi: 10.3791/52806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davies SJ, Goucher DR, Doller C, et al. Robust regeneration of adult sensory axons in degenerating white matter of the adult rat spinal cord. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19(14):5810–22. doi: 10.1523/JNEUROSCI.19-14-05810.1999. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gensel JC, Nakamura S, Guan Z, et al. Macrophages promote axon regeneration with concurrent neurotoxicity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29(12):3956–68. doi: 10.1523/jneurosci.3992-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lerch JK, Martinez-Ondaro YR, Bixby JL, et al. cJun promotes CNS axon growth. Molecular and cellular neurosciences. 2014;59:97–105. doi: 10.1016/j.mcn.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen Q, Riedl KM, Cole RM, et al. Egg yolks inhibit activation of NF-kappaB and expression of its target genes in adipocytes after partial delipidation. Journal of agricultural and food chemistry. 2015 doi: 10.1021/jf5056584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hernandez-Pedro N, Granados-Soto V, Ordonez G, et al. Vitamin A increases nerve growth factor and retinoic acid receptor beta and improves diabetic neuropathy in rats. Translational research : the journal of laboratory and clinical medicine. 2014;164(3):196–201. doi: 10.1016/j.trsl.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 37.Peeraully MR, Jenkins JR, Trayhurn P. NGF gene expression and secretion in white adipose tissue: regulation in 3T3-L1 adipocytes by hormones and inflammatory cytokines. American journal of physiology Endocrinology and metabolism. 2004;287(2):E331–9. doi: 10.1152/ajpendo.00076.2004. [DOI] [PubMed] [Google Scholar]
- 38.Bullo M, Peeraully MR, Trayhurn P, et al. Circulating nerve growth factor levels in relation to obesity and the metabolic syndrome in women. Eur J Endocrinol. 2007;157(3):303–10. doi: 10.1530/EJE-06-0716. [DOI] [PubMed] [Google Scholar]
- 39.Molotkov A, Duester G. Genetic evidence that retinaldehyde dehydrogenase Raldh1 (Aldh1a1) functions downstream of alcohol dehydrogenase Adh1 in metabolism of retinol to retinoic acid. The Journal of biological chemistry. 2003;278(38):36085–90. doi: 10.1074/jbc.M303709200. [DOI] [PubMed] [Google Scholar]
- 40.Overman JJ, Clarkson AN, Wanner IB, et al. A role for ephrin-A5 in axonal sprouting, recovery, and activity-dependent plasticity after stroke. Proc Natl Acad Sci U S A. 2012;109(33):E2230–9. doi: 10.1073/pnas.1204386109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hernandez-Miranda LR, Cariboni A, Faux C, et al. Robo1 regulates semaphorin signaling to guide the migration of cortical interneurons through the ventral forebrain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(16):6174–87. doi: 10.1523/JNEUROSCI.5464-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Janes PW, Saha N, Barton WA, et al. Adam meets Eph: an ADAM substrate recognition module acts as a molecular switch for ephrin cleavage in trans. Cell. 2005;123(2):291–304. doi: 10.1016/j.cell.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 43.Hattori M, Osterfield M, Flanagan JG. Regulated cleavage of a contact-mediated axon repellent. Science. 2000;289(5483):1360–5. doi: 10.1126/science.289.5483.1360. doi. [DOI] [PubMed] [Google Scholar]
- 44.Chang TM. Pharmaceutical and therapeutic applications of artificial cells including microencapsulation. Eur J Pharm Biopharm. 1998;45(1):3–8. doi: 10.1016/S0939-6411(97)00117-3. S0939-6411(97)00117-3 [pii] [DOI] [PubMed] [Google Scholar]
- 45.Chang TMS. Semipermeable microcapsules. Science. 1964;146:524–5. doi: 10.1126/science.146.3643.524. doi. [DOI] [PubMed] [Google Scholar]
- 46.Cortez M, Singleton JR, Smith AG. Glucose intolerance, metabolic syndrome, and neuropathy. Handbook of clinical neurology. 2014;126:109–22. doi: 10.1016/B978-0-444-53480-4.00009-6. [DOI] [PubMed] [Google Scholar]
- 47.Giordano A, Cesari P, Capparuccia L, et al. Sema3A and neuropilin-1 expression and distribution in rat white adipose tissue. J Neurocytol. 2003;32(4):345–52. doi: 10.1023/B:NEUR.0000011328.61376.bb. [DOI] [PubMed] [Google Scholar]
- 48.Kang SK, Shin MJ, Jung JS, et al. Autologous adipose tissue-derived stromal cells for treatment of spinal cord injury. Stem Cells Dev. 2006;15(4):583–94. doi: 10.1089/scd.2006.15.583. [DOI] [PubMed] [Google Scholar]
- 49.Kir S, White JP, Kleiner S, et al. Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature. 2014;513(7516):100–4. doi: 10.1038/nature13528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Randall JC, Winkler TW, Kutalik Z, et al. Sex-stratified genome-wide association studies including 270,000 individuals show sexual dimorphism in genetic loci for anthropometric traits. PLoS genetics. 2013;9(6):e1003500. doi: 10.1371/journal.pgen.1003500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeggini E, Scott LJ, Saxena R, et al. Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat Genet. 2008;40(5):638–45. doi: 10.1038/ng.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao J, Bradfield JP, Zhang H, et al. Examination of all type 2 diabetes GWAS loci reveals HHEX-IDE as a locus influencing pediatric BMI. Diabetes. 2010;59(3):751–5. doi: 10.2337/db09-0972. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.