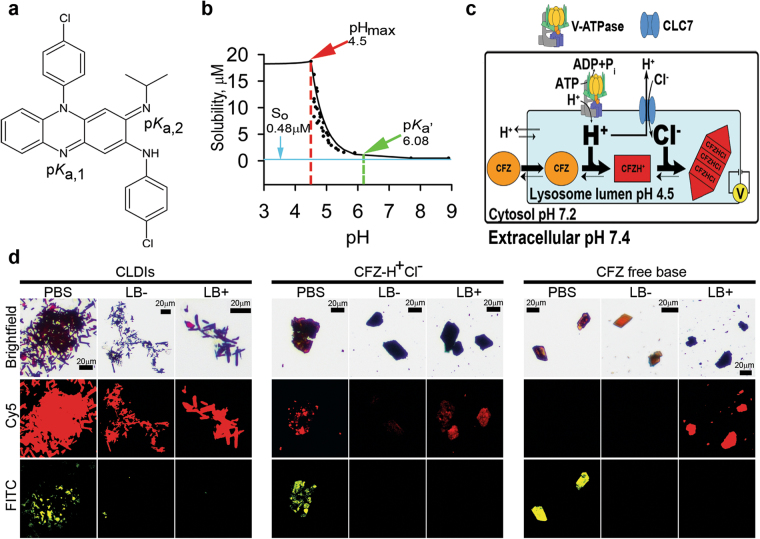
Figure 1.
Chemical characterization of CFZ. (a) Chemical structure of clofazimine (CFZ) with its two protonation sites and corresponding predicted (chemi-informatic) pKa values (pKa,1 = 2.31 and pKa,2 = 9.29). (b) CFZ-H+Cl− solubility-pH study revealed the solution pH dependence of the stabilization of the free base versus salt form of the drug with respect to its solubility parameters; which include the intrinsic free base solubility (So), apparent pKa,2 (pKa’), and pHmax. (c) Illustration showing the cellular and subcellular accumulation of free base CFZ, its subsequent protonation (CFZH+), and ion-ion interaction of CFZH+ and cellular Cl− to form CFZ-H+Cl−. This phenomenon depends on the drug’s intrinsic solubility properties as well as the cellular pH and Cl− levels, which are primarily regulated by membrane proteins: proton-pump known as V-ATPase and Cl−/H+ antiporter known as CLC7. (d) Stability of CLDIs, CFZ-H+Cl−, and free base CFZ in PBS (pH 7.4), lysosomal buffer without sodium chloride (LB−, pH 4.5), and lysosomal buffer with 100 mM sodium chloride (LB+, pH 4.5) was monitored via brightfield and fluorescence microscopy.