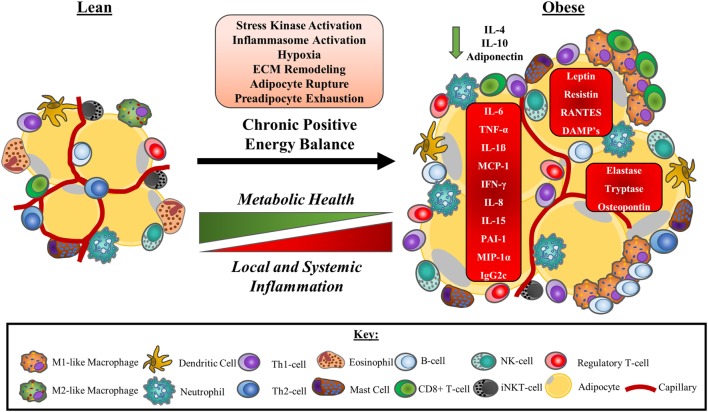
Figure 3.
A model of obesity-driven immunological changes within adipose tissue. Under a chronic state of positive energy balance, adipose tissue undergoes a multitude of changes that include the hypertrophic expansion of adipocytes without concurrent angiogenic responses. The consequences of these adaptations include local tissue hypoxia as a result of reduced tissue blood flow and impaired oxygen delivery. The rapid expansion of adipocytes also leads to unstable adipocytes prone to rupturing, releasing their lipid contents. This instability is further exacerbated by an exhaustion of the preadipocytes required to facilitate adipose tissue expansion. The net result of these physiological changes to adipocytes is the release of DAMPs and other signalling molecules that initiate stress kinase activation and promote monocyte infiltration to manage the remodelling of the ECM. Neutrophils are the first cells to accumulate in adipose tissue, followed by a range of other immune cells, most notably, B-cells, CD8+ T-cells, and M1-like/intermediate, or “double-negative” (CD11c−CD206−) macrophages that surround unstable adipocytes in crown-like structures. As a consequence, a Th-1 cell and M1-like macrophage secretion profile predominated and some anti-inflammatory immune cells such as eosinophils and iNKT-cells leave the tissue. Other anti-inflammatory cells, including regulatory T-cells, may increase to compensate. Pro-inflammatory cytokines and adipokines dominate the tissue microenvironment potentiating the activation of stress kinases and inflammasomes. Local inflammation reduces adipose tissue insulin sensitivity, impairing metabolic health and promoting ectopic lipid deposition. Pro-inflammatory adipose tissue contributes to the chronic, low-grade inflammation characteristic of obesity, and metabolic disease. Abbreviations: CD, cluster of differentiation marker; DAMP, damage-associated molecular pattern; ECM, extracellular matrix; IFN-γ, interferon gamma; Ig, immunoglobulin; IL, interleukin; iNKT-cell, invariant natural killer T cell; MCP-1, monocyte chemotactic protein 1 (CCL-2); MIP-1α, macrophage inflammatory protein 1 alpha (CCL-3); PAI-1, plasminogen activator inhibitor 1; RANTES, regulated on activation, normal T-cell expressed and secreted (CCL-5); Th cell, helper T cell; TNF-α, tumour necrosis factor-α.