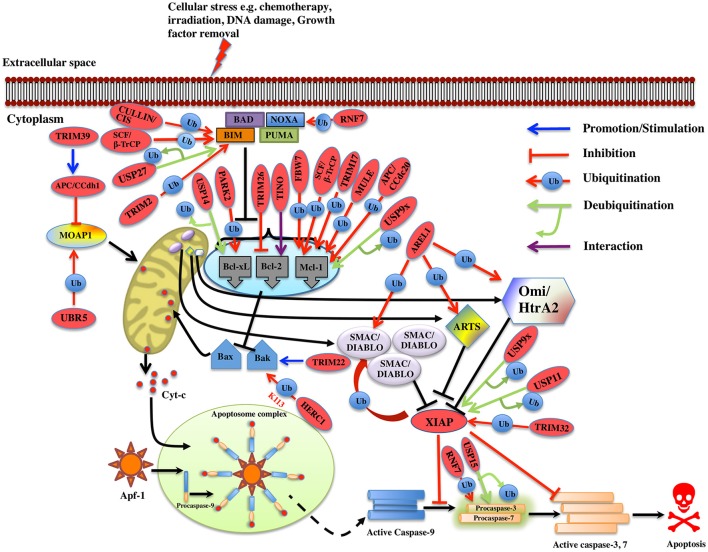
Figure 3.
Regulation of Intrinsic pathway by E3 ligases and DUBs. Multiple signals such as DNA damage, unfolded protein response, cytokine deprivation, free radical generating compounds, removal of nutrients, oxygen or growth factors, and endoplasm reticulum stress etc. contributes in the activation of intrinsic or the mitochondrial apoptotic pathway. Following stress, two proapoptotic Bcl-2 (B-cell lymphoma 2) proteins- Bax (Bcl-2 associated X protein) and Bak (Bcl-2 homologous antagonist killer) are activated upon structural changes. They move to the mitochondria, homodimerizes, and generate pores onto the mitochondrial surface, which enhances the membrane permeabilization and cytochrome c then starts to releases. In the presence of ATP, cytochrome c associates with Apaf-1, leading to self-oligomerization of Apaf-1 via CED-4 domains. This leads to the exposure of CARDs of Apaf-1. When these CARDs domain of Apaf-1 and caspase-9 binds, an apoptosome is formed. Dimerization of procaspase-9 forms an active caspase-9, which further activates the caspase-3 and 7 to commit cell death. Bcl-2 family proteins are categorized as antiapoptotic [Bcl-2, Bcl-xl (Bcl-extra-large), Bcl-w, Mcl-1 (Myeloid leukemia cell differentiation 1), Bcl-b and A1], proapoptotic (Bax, Bak, and Bok (Bcl-2 related ovarian killer)] and regulatory BH3-only proteins [Bad (Bcl-2 antagonist of cell death), Bik (Bcl-2-interacting killer), Bid (BH3-interacting domain death agonist), Bim (Bcl-2 interactor mediator of cell death), Bmf (Bcl-2-modifying factor), Noxa, and Puma (p53 upregulated modulator of apoptosis)], Antiapoptotic proteins suppress the apoptotic signals by limiting Bax and Bak thus blocking cytochrome-c release. In case of cellular stress, Bim and Noxa counteract the effect of antiapoptotic proteins. BH3-only proteins release Bax-Bak from inhibition thus promoting Momp (Mitochondrial outer membrane potential) and apoptosis. The IAPs (inhibitors of apoptosis) are the negative regulators of the intrinsic pathway. XIAP (X-linked Inhibitor of Apoptosis Protein) is one of the IAP to be seen upregulated in cancer and is an inhibitor of caspase. Activation of the intrinsic apoptotic pathway leads to the release of Smac/Diablo (Second mitochondrial activator of caspases or direct IAP binding protein with low pI), Omi/HtrA2 (Omi stress-regulated endoprotease or High-temperature requirement A2) and Arts (Apoptosis-related protein in the TGF-beta signaling pathway) into the cytosol. Smac/Diablo and Omi/HtrA2, when released into cytosol upon stimuli, use their IBM (IAP-binding motif) domain to bind to the BIR2 and BIR3 domains of the IAPs. They prevent the XIAPs ability to inhibit caspases and thus mediate apoptosis. Arts interact with residues of the BIR3 domain of XIAP distinct from Smac/Diablo and Omi/HtrA2 suggesting it to antagonize XIAP in a different way. Several E3 ligase and DUBs involved at different stages of the pathway are shown as red ovals.