Abstract
Objective(s):
Micelles have been studied as nanoparticulate drug delivery systems for improving the topical ocular delivery of hydrophobic drugs. The objective of this study was to develop and characterize dexamethasone-loaded polycaprolactone-polyethylene glycol-polycaprolactone (PCL-PEG-PCL) micelles to improve patient compliance and enhance the ocular bioavailability of poorly water-soluble drugs.
Materials and Methods:
The PCL-PEG-PCL copolymers were synthesized via the ring opening polymerization of ε-caprolactone in the presence of PEG. The resulting purified copolymers were characterized by GPC, NMR, FTIR, XRD and DSC. The critical micelle concentrations (CMCs) of the mentioned copolymers were determined. Dexamethasone was loaded into polymeric micelles by film hydration method, and dexamethasone-loaded micelles were characterized by TEM and DLS. Drug release kinetics and ex vivo corneal permeability were also determined.
Results:
The CMC of the synthetized copolymers was approximately 0.03 mg/ml. Aqueous solutions of the resulting copolymers (400 mg/ml) rapidly formed a gel in situ at 34°C. The TEM results exhibited the successful formation of spherical micelles. The size of the prepared micelles was approximately 40 nm. Formulated micelles sustained the release of the incorporated dexamethasone for 5 days.
Conclusion:
Data from ex vivo permeability tests indicated that PCL-PEG-PCL micelles can be suitable candidates for the ocular delivery of dexamethasone and, likely, other hydrophobic drugs.
Keywords: Block copolymer, Critical micelle concentration, Dexamethasone, Micelle, Ocular drug delivery
Introduction
Anterior uveitis is the most prevalent form of ophthalmic inflammation that anatomically involves inflammation of the iris alone (iritis), the anterior part of the ciliary body (anterior cyclitis) or both structures (iridocyclitis) (1). Patients who suffer from anterior uveitis usually repine of pain, redness, unclear vision, and photophobia (2).
Corticosteroids are the drug of choice in the treatment of anterior uveitis (1). Eye drops and suspensions are often used for the topical administration of ocular drugs (3). These approaches are probably used because of their ease of administration and patient compliance (4-6). A drawback of these dosage forms is that as the dosage form is introduced into the cul-de-sac, the tear film dilutes, and lacrimal-nasal drainage rapidly drains away the existing active constituent (3). The drainage rate constant from the pre-corneal cavity is 1.45 min -1, so the rate of drug loss from the eye surface can be 500 to 700 times more than the rate of drug absorption into the anterior segment (7) ; therefore, less than 5% of the topically administered dose reaches the intraocular tissues (7-10).
The commonly applied volume for topically administered formulations is usually less than 40–50 µl to counteract the drug loss due to reflux tear secretion and nasolacrimal drainage. A low instillation volume necessitates steroidal drugs to be dissolved in aqueous solution at higher concentrations to achieve a therapeutic drug level in the eye. However, steroids are hydrophobic in nature and cannot be dissolved at high concentrations in aqueous solution (8). Currently, biomaterials such as polymer-drug conjugates (11), nanoparticles (12, 13), micelles (14, 15), and liposomes (16), are used as pharmaceutical vehicles for solubilizing hydrophobic drugs. Micelles considered for ocular drug delivery can be categorized as polymeric (including block copolymers), surfactant, or polyionic complex (PIC) micelles (17).
Amphiphilic block copolymers undergo phase separation in aqueous media as a result of chain association above a certain concentration, which is called the critical micelle concentration (CMC), and form nano-sized (10-100 nm) colloidal dispersions with a hydrophobic core and a hydrophilic shell (18, 19). Typical surfactant micelles are defined by higher CMC values where there is a dynamic balance between micellar assemblies and unimers in the solution. The micellar assemblies that are formed by surfactants are weak and may dissociate into unimers upon dilution. In contrast, polymeric micelles show lower CMCs and are more stable against dilution (17, 18).
Boddu et al. formulated dexamethasone (DEX)-loaded PLGA nanoparticles using both dialysis and O/W solvent evaporation methods (20). Gómez-Gaete et al. investigated several parameters to enhance the DEX entrapment in PLGA nanoparticles. They reached the highest loading amount of 230 µg using 100 mg of PLGA and 10 mg of DEX by the solvent evaporation technique (21). Recently, Vaishya et al. considerably improved DEX entrapment into di-block polymeric micelles using modified film hydration method to overcome the undesirable effect of polymer crystallization (8).
Since the core of micelles can be utilized to incorporate poorly water-soluble drugs, it seems that even better loading properties can be achieved by introducing other hydrophobic blocks to the existence di-block copolymers. So in this contribution, our goal was to encapsulate DEX in polycaprolactone-polyethylene glycol-polycaprolactone (PCL-PEG-PCL) micelles as a promising vehicle with sustained release properties. In this way, a micellar delivery system with PCL-PEG-PCL was synthesized, and the release profile and in vitro corneal permeation of DEX from the prepared micelles were studied.
Materials and Methods
Chemicals and materials
Dexamethasone base was procured from Alborz bulk pharmaceutical co. (Tehran, Iran).
ε-caprolactone, stannous octoate (Sn(Oct)2), and pyrene were purchased from Sigma-Aldrich (St Louis, USA). PEG1500, di-sodium hydrogen phosphate, potassium di-hydrogen phosphate, sodium chloride, calcium chloride, potassium chloride dihydrate, sodium hydrogen-carbonate, and sodium di-hydrogen phosphate dihydrate, were obtained from Merck (Darmstadt, Germany). Acetonitrile, tetrahydrofuran, dichloromethane, and diethyl ether were bought from Scharlau (Barcelona, Spain), and Maxidex® (dexamethasone 0.1%) sterile ophthalmic suspension was procured from Alcon® (Hünenberg, Switzerland SA). All solvents and reagents utilized in this study were of analytical grade, and deionized water was used during the HPLC procedure.
Experimental design
Synthesis of PCL-PEG-PCL tri-block copolymer
The PCL-PEG-PCL copolymer was synthesized by ring-opening polymerization method as described in the literature (22-24). Briefly, 6 g of PEG1500 was heated at 110 °C for 15 min in a two-necked, round-bottom flask to remove the water adsorbed to the polymer. Then, 12 ml of ε-caprolactone and 0.5% (w/w) of Sn(Oct)2 (as catalyst) were added to the reaction vessel, the temperature was raised to 130 °C and the mixture was stirred under nitrogen atmosphere for 24 hr. Subsequently, the product was dissolved in dichloromethane and precipitated in cold diethyl ether, and the copolymer was dried under vacuum for 48 hr at 50 °C (Figure 1).
Figure 1.

Synthesis scheme of the PCL-PEG-PCL copolymer
Characterization of PCL-PEG-PCL copolymer
X-ray diffraction (XRD)
XRD analysis was carried out using a D500 X-ray diffractometer (Siemens, Germany) equipped with a position-sensitive detector allowing all angles between 4 °C and 70 °C to be read simultaneously at a scan rate of 1°/min. Cu Kα was used as the radiation source (λ=1.54Å).
Differential scanning calorimetry (DSC)
The thermal behaviors of DEX, blank copolymer, DEX/copolymer physical mixture, and dried DEX/ copolymer film were characterized by differential scanning calorimeter (Shimadzu, Japan). A total of 2 mg of each sample was placed in an aluminum pan and heated from 25 °C to 300 °C at a heating rate of 10 °C/min.
Fourier transform infrared spectroscopy (FTIR)
Fourier transform infrared spectra were recorded with a computerized ATR-FTIR spectrometer (Bruker, Tensor 27, USA). The scan was carried out in the range of 4000–400 cm−1 at a resolution of 1 cm-1.
Gel permeation chromatography (GPC)
An Agilent GPC-Addon apparatus (CA, USA), equipped with a RID-A refractive index signal detector (Agilent Technology, CA, USA) and coupled to a Plgel column (10 µm, 300×7.5 mm-Agilent Technology, CA, USA), was used to determine the number (Mn) and molecular weight (Mw) averages as well as the poly-dispersity of the PCL-PEG-PCL tri-block copolymers. The mobile phase was tetrahydrofuran (THF) with a flow rate of 1.000 ml/min. The injection volume was 20.000 µl of stock solution (1.000 g/l), and polystyrene monodisperse was used as a standard.
Transmission electron microscopy (TEM)
The morphology of the prepared PCL-PEG-PCL micelles was observed by a Leo 906E transmission electron microscope (Zeiss, Germany) at 100 kV. A drop of the polymeric micelle solution was placed on a carbon-coated copper grid. The sample was negatively stained with phosphotungstic acid and dried completely at room temperature before observation.
Nuclear magnetic resonance (NMR)
Proton nuclear magnetic resonance spectroscopy (1H-NMR) was carried out to determine the structure and average molecular weight of the copolymer using a Bruker Spectrospin 400 MHz spectrometer (Varian, Switzerland) in CDCL3.
The methylene peak of the homosequences of the PEG CH2CH2O unit at 3.64 ppm, the methylene peak of the CH2OOC unit in the PCL blocks at 4.06 ppm and the methylene peak of OCH2 in the PEG end unit at 4.23 ppm in the 1H-NMR spectra were used to calculate the number average molecular weight (Mn) and the PCL/PEG ratio by using Equations (1-3):



where Ia, Id, and If are integral intensities of methylene protons of the CH2OOC, OCH2 and CH2CH2O units, respectively, and x and y are the corresponding block numbers of PCL and PEG, respectively, in the PCL-PEG-PCL copolymer (25).
Measurement of sol-gel transition temperature
The sol-gel transition study was carried out using the test tube inverting method. A total of 400 mg of the synthesized copolymer was dispersed in 1 ml of distilled water in 10 ml vials. These dispersions were heated to 60 °C until the copolymer was melted, followed by quenching in an ice bath for one minute. The prepared samples were examined to determine the phase transition temperature by heating the vials from room temperature to 50 °C in a shaker incubator. The vials were kept at each temperature for 3 min and then removed from the incubator and tilted 90 °C. The samples with no flow within 30 sec were considered gel.
CMC assay
The CMC of the PCL-PEG-PCL micelles was determined using pyrene as a fluorescent probe. A series of aqueous solutions with various concentrations of the copolymer ranging from 6×10-5 to 0.25 mg/ml was obtained. A known amount of pyrene was added to each of this series to give a final concentration of 6.2×10-6 M of pyrene to each solution. These solutions were kept at 36 °C in the dark and under slight agitation for 20 hr. The samples were then excited at 334 nm in a Jasco FP-750 spectrofluorimeter (Kyoto, Japan) equipped with a 150 W xenon lamp, and the emissions were recorded in the 340-450 nm wavelength range. The slit widths for both emission and excitation were 3 nm. All measurements were done at room temperature. The ratio of the first and third intensity peaks was plotted against the log concentration of the copolymer to assess the CMC.
Rheological studies
Rheological experiments were performed using a cone and plate viscometer (HAAK, Germany) at 25 °C and 34 °C. Approximately 2 to 3 drops of the sample (containing 20% w/v copolymer) were placed on the plate of the rheometer using a sampler, and the knob was released to bring the plate into contact with the tip of the cone. Measurements were carried out under the various shear moduli (n= 1, 2, 4, 8, 16, 32, 64, 128, 256), and the stress moduli were read through the display device. The shear rate in sec-1, shear stress in dyne/cm2, and viscosity in Cp were specified from the instrument reading and using special equations. The data obtained were plotted as shear stress and viscosity versus shear rate.
Preparation of DEX-loaded micelles
DEX-encapsulated micelles were prepared by the film hydration method (Figure 2). Briefly, measured amounts of the PCL-PEG-PCL copolymer (200 mg) and DEX (10 mg) were dissolved in 2 ml of THF in test tubes. The organic solvent was completely evaporated to generate polymer/drug matrix films. Then, 1 ml of deionized water was added to each film and stirred continuously until it was completely dispersed. These dispersions were heated to 60 °C until the copolymer was melted, followed by quenching in an ice bath for one minute (both heating and quenching were done under magnet stirring). The prepared formulations were filtered through 0.22 µm syringe-filters (Millipore™, Billerica, MA) to separate undissolved drug and aggregated polymers and were then evaluated for DEX solubility by reverse phase high-performance liquid chromatography (RP-HPLC). The loaded drug was released by diluting a certain volume of formulation in THF, and the amount of DEX in the diluted solution was determined using a calibration curve of DEX in the THF/acetonitrile mixture.
Figure 2.

Micelle preparation procedure via film hydration method
Micelle size, polydispersity, and zeta potential
The mean particle size and polydispersity index (PDI) were determined by dynamic light scattering using a Zetasizer (Zetasizer Nano ZS, Malvern Instruments Ltd, Worcestershire, UK) at room temperature. The amount of electric charge at the surface of the micelles, which indicates the physical stability of colloidal dispersions, was determined by measuring the zeta potential. The formulations were prepared in 1.5 ml water without any further dilution.
In vitro release studies
The release profile of DEX from the PCL-PEG-PCL micelles was determined using the in vitro release study in simulated tear fluid (STF). The DEX-loaded micelles were prepared as described previously. A total of 200 µl of the DEX marketed eye drop or DEX-loaded micelles was poured into a dialysis bag (MW 2,000 Da). The bag was tied at both ends and immersed in 20 ml of STF containing 0.5% w/w Tween-80 at 34 °C. The release medium was replaced with fresh STF at predetermined time points. The amount of DEX released was quantified by RP-HPLC. The release study was carried out in triplicate.
Statistical analyses of data were carried out by comparing the dissolution efficiency (DE), mean dissolution time (MDT), and difference factor (f1). DE, MDT, and f1 were computed using equations 4 (26), 5 (27), and 6 (28), respectively:

Where y is the percentage of the drug dissolved at time t,
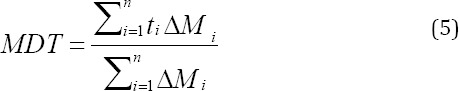
Where i is the sample number, n is the number of dissolution samples, ti is the time at midpoint between t and ti-1 and ∆Mi is the additional amount drug dissolved between t and ti-1,
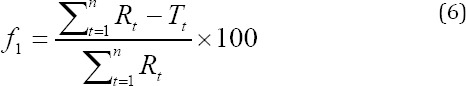
Where Rt and Tt are the percentages of the drug dissolved for the reference and test formulation at each time point.
Drug release kinetics
To determine the kinetics of DEX release from the prepared micelles, release data were applied to different mathematical models, including zero-order, first-order, Higuchi, Hixson Crowell, and Korsmeyer-peppas. The parameters of the models were obtained by linear regression. The model that had the highest regression coefficient (R2) and the lowest mean percent error (MPE%) was chosen as the best fit to determine the release kinetics of DEX from the prepared formulations.
Ex vivo permeation studies
The ex vivo permeation study of the DEX-loaded PCL-PEG-PCL micelles was performed in comparison with marketed eye drop (0.1% w/v) by mounting the freshly prepared bovine cornea on a Franz-type vertical diffusion cell with superficial epithelial cells facing the donor compartment. The receptor compartment was filled with phosphate-buffered saline (PBS: pH=7.4) and the chambers were clamped together. A total of 300 µl of the DEX marketed eye drop or DEX-loaded micelles was added to the donor compartment, and the open side of the donor chamber was sealed with Parafilm®. At predetermined time intervals, 300 µl of the samples was withdrawn from the receptor compartment and replaced with an equivalent volume of fresh PBS buffer. The samples were analyzed using RP-HPLC at 254 nm. All trials were performed in triplicate under sink conditions at 32 °C for 24 hr.
Statistical analyses were carried out using an independent Student’s t-test (SPSS software). Differences were considered to be significant at a level of P<0.05.
Histopathological evaluation of eye tissue
The histopathological effects of the thermogeling formulations were assessed. The tissue was fixed using formalin 10%, processed as is routine, and embedded in paraffin. Paraffin was cut into slices on the glass slides, and the sections were stained with hematoxylin and eosin. An optical microscope recorded any damage caused within the tissue.
HPLC method
A liquid chromatographic system (Knauer, Germany) consisting of a Knauer K1000 pump equipped with a Rheodyne (Cotati, CA) injector, a variable wavelength ultraviolet spectrophotometric detector (Knauer smartline 2500) set at 254 nm and a reverse phase C18 column (250×4.6 mm, 5 µm) were used to determine the amount of DEX. The mobile phase was composed of acetonitrile, and deionized water (50:50, v/v), which was degassed before its use, was used as the eluting solvent at a flow rate of 1 ml/min.
Results
The tri-block copolymer of PCL-PEG-PCL was synthesized via the ring-opening copolymerization of ε-caprolactone in the presence of PEG as an initiator and Sn(Oct)2 as a catalyst. The chemical structure, molecular weight and molecular weight distribution of the copolymer obtained were determined using FT-IR, 1H-NMR, and GPC analysis, as summarized in Table 1. All the results indicated the successful synthesis of the copolymer.
Table 1.
Characteristics of the synthesized PCL-PEG-PCL copolymer determined by various methods
| Copolymer | PCL/PEGa (Theoretical) | Total Mna (Theoretical) | PCL/PEGb (Calculated) | Total Mnb (Calculated) | Total Mnc (Calculated) | PDIc |
|---|---|---|---|---|---|---|
| PCL-PEG-PCL | 2:1 | 4500 | 1.73:1 | 4448 | 8465 | 1.33 |
Theoretical value, calculated according to the feed ratio;
Determined from 1H-NMR results;
Calculated from GPC results
Physicochemical characterization
FTIR analysis
The FT-IR spectrum of the synthesized copolymer is displayed in Figure 3. The strong bond appearing at 1724.09 cm-1 is related to the carbonyl stretching vibration of PCL, which supports the copolymer production (29). The C–H stretching bonds of polyethylene oxide and PCL were observed at 2868.49 and 2940.60 cm-1, respectively (30). The signals appearing at 1101.35 and 1181.81 cm-1 are related to the stretching vibrations of the C-O-C units, and the band observed at 1274 cm−1 is attributed to the presence of the -COO-stretching vibration (31).
Figure 3.

FTIR spectroscopy of dexamethasone (a), PEG1500 (b), blank copolymer (c), dried dexamethasone/copolymer film (d)
NMR analysis
The 1H-NMR spectrum of the PCL-PEG-PCL copoly-mer is presented in Figure 4. The signals at 1.38, 1.65, 2.31 and 4.06 ppm are attributed to the methylenes of (CH2)3, OCCH2, and CH2OOC in PCL blocks, respectively. The sharp peak at 3.65 ppm is assigned to the methy-lene protons of homosequences of the PEG CH2CH2O units, and the very weak peaks at 4.22 and 3.83 ppm are attributed to the methylene protons of CH2CH2O that are linked to PCL blocks.
Figure 4.

1H-NMR spectrum of the PCL-PEG-PCL copolymer.
GPC analysis
Figure 5 depicts the GPC chromatogram of the synthesized copolymer. As shown, the GPC curve is symmetric that indicates the low polydispersity of the copolymer. No shoulder peaks related to the PCL homopolymer were observed.
Figure 5.
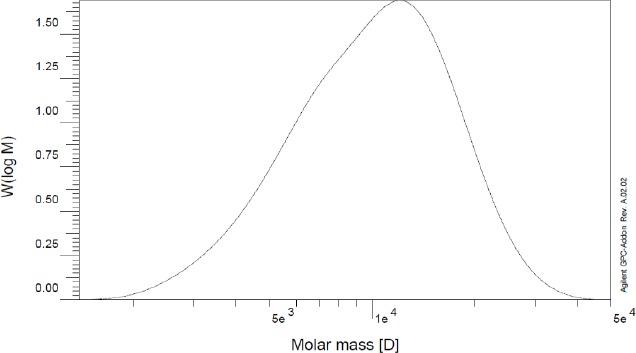
GPC chromatogram of the PCL-PEG-PCL copolymer
XRD analysis
The XRD diffractograms of DEX, PEG1500, blank copolymer, DEX/copolymer physical mixture, and dried DEX/copolymer film are shown in Figure 6. The blank copolymer exhibited characteristic peaks at 2θ 21.5 °C and 23.78 °C. Diffraction patterns of the physical mixture showed very small peaks of the drug at 13.64 °C, 16.15 ° C, and 17.80 ° C.
Figure 6.

XRD diffractogram of dexamethasone (a), PEG1500 (b), dried dexamethasone/copolymer film (c), blank copolymer (d), and physical mixture (e)
DSC analysis
Figure 7 shows DSC thermograms of DEX, PEG1500, blank copolymer, DEX/copolymer physical mixture, and dried DEX/copolymer film. The DEX thermogram exhibited a melting peak at 253.09 °C. Endothermic peaks located at 38.27 °C and 57.58 °C in the thermogram of the copolymer were related to the melting points of PEG and PCL blocks, respectively. The thermogram of the dried film showed endothermic peaks at 37.45°C and 53.47°C related to the melting point of the recrystallized polymer.
Figure 7.
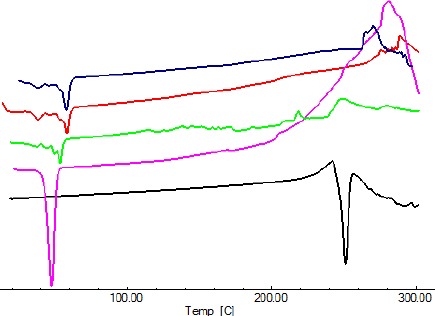
DSC thermogram of dexamethasone (a), PEG1500 (b), dried dexamethasone/copolymer film (c), blank copolymer (d), and physical mixture (e)
Sol-gel transition studies
The synthetized PCL-PEG-PCL copolymer displayed a temperature dependent reversible sol-to-gel transition in water. The polymer solution (400 mg/ml) was in a free-flowing clear sol state at ambient temperature. Increasing the temperature to 34 °C made the solution convert to a clear gel (lower transition temperature). The clear gel was converted to an opaque gel and then an opaque sol upon further increasing the temperature (upper transition temperature). As can be seen, the gel window of the synthetized copolymer included 34 °C, which is the temperature of the eye surface.
Rheological studies
Viscosity studies using a cone and plate viscometer were also carried out to further determination of the rheological properties of the prepared polymer solution. Figure 8A displays the graph of shear stress versus shear rate at room temperature (a) and at ocular temperature (b). It is clear that the sample tested displayed non-Newtonian pseudo-plastic behavior, as determined by a down-facing concave. The graph of apparent viscosity, which is the slope of the graph of shear stress vs shear rate at every point, against the shear rate is shown in Figure 8B. As shown, the viscosity of the copolymer solution increased upon exposure to ocular temperature.
Figure 8.
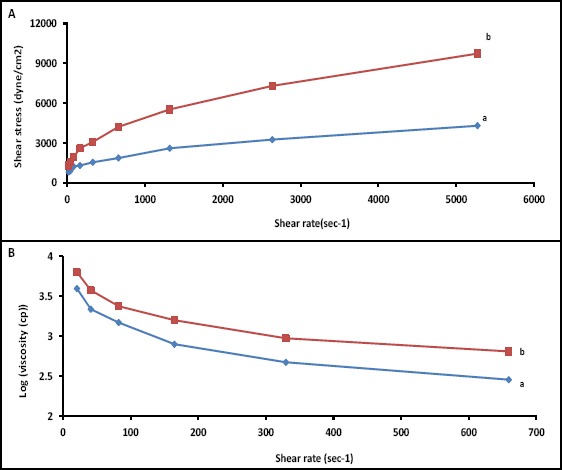
A) Shear stress versus shear rate of the solution containing 40% w/v copolymer in water at a) 25 °C and b) 34 °C; B) Apparent viscosity (log scale) versus shear rate of the solution containing 40% w/v copolymer in water at a) 25 °C and b) 34 °C.
Size and morphology studies
Micellar size and size distribution were determined for blank and drug-loaded copolymer solutions by DLS. The outcomes are reported in Table 2.
Table 2.
Summary of the micellar formulation properties including dexamethasone solubility, micelle size, PDI, and zeta potential
| Formulation code | Polymer (mg) | aDEX (mg) | Solubility±SD (mg/ml) | Size±SD (nm) | PDI | Zeta Potential (mV±SD) |
|---|---|---|---|---|---|---|
| Micelles | 200 | 10 | 1.17±0.16 | 40.04±2.42 | 0.358 | -1.70±1.39 |
| Micelles Blank copolymer | 200 | - | 37.24±3.80 | 0.319 | -0.178±0.41 |
dexamethasone
The incorporation of drug into the micelles led to a slight increase in size. The size of drug-loaded micelles was 40.04 nm with unimodal distribution. The PDI of these formulations was 0.358, indicating a narrow size distribution. The morphology of the obtained micelles was studied by TEM (Figure 9) and demonstrated that the micelles were spherical in shape. The aqueous solubility of DEX (0.1mg/ml) increased and reached to 1.17 mg /ml in presence of micelles.
Figure 9.
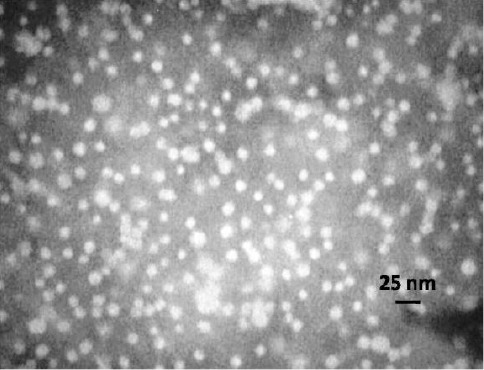
TEM image of the PCL-PEG-PCL micelles
CMC assay
The CMC of the PCL-PEG-PCL micelles was determined using pyrene as a fluorescent probe. Figure 10B depicts the plot of the intensity ratios of I373/I384, which were recorded from the excitation of the samples at 334 nm as a function of the concentration of the PCL-PEG-PCL aqueous solutions on a logarithmic scale. As can be seen, the plot of I373/I384 vs. log C is flat at low PCL-PEG-PCL concentrations. A significant decrease in the intensity ratio occurs above a certain concentration, indicating a change in the pyrene micro-environment caused by its transfer from a polar environment into the hydrophobic micelle cores; meantime the aggregates begin to form. The CMC was determined from the abscissa of the intersection of the straight line at the low concentration range and the horizontal line at the high concentration range. The CMC obtained was approxi-mately 0.03 mg/ml.
Figure 10.
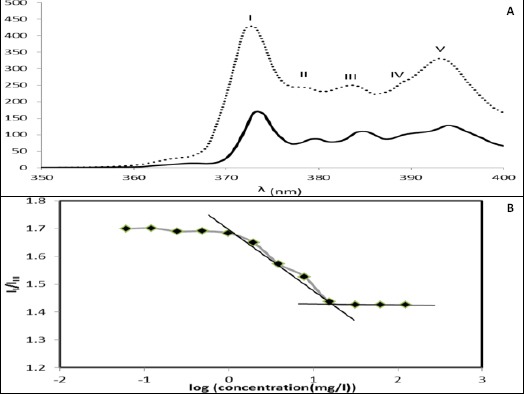
A) Fluorescence emission spectrum of pyrene (6.2× 10-5 M) in polymer solution of 10-3 mg/ml (….) (i.e., below the CMC) and 10-1 mg/ml (—) (i.e., above the CMC); B) Plot of the intensity ratio (II/IIII) of pyrene emission spectra as a function of PCL-PEG-PCL concentration in logarithmic scale
In vitro release studies
The in vitro release profile of DEX from the micelles in STF containing 0.5% Tween 80 is shown in Figure 11. The formulations exhibited a sustained release pattern with a very low burst release (7.89% after 2 hr).
Figure 11.

Cumulative percent release of dexamethasone from the micelles and marketed drop
Table 3 compares various release characteristics of DEX from the micelles and marketed drop. As shown, micelles had a DE of 81.48. The marketed drop had a higher release rate (MDT=527.51 min) (P<0.05), and the increase in DE (93.11) was significant compared to that of the micelles (P<0.05). The difference factor also suggested that there was a significant difference in the release profiles of these two drug forms (f1 = 16.24).
Table 3.
Comparison of various release characteristics, Flux and permeability coefficient of dexamethasone from the micelles, and marketed drop
| Formulation code | aRel2 (%±SD) | bRel24 (%±SD) | cRel120 (%±SD) | dDE | eMDT (min) | fFlux (mg/cm2.min) *10-4±SD*10-7] | gPapp (cm/s) *10-6±SD*10-7] | Lag time (min) |
|---|---|---|---|---|---|---|---|---|
| Micelles | 7.89±0.37 | 71.19±9.10 | 100.17±2.91 | 81.48 | 1343.22 | 0.23±0.57 | 0.33±0.82 | 135 |
| Marketed drop | 24.57±7.50 | 92.48±3.40 | - | 93.11 | 527.51 | 0.1±0.00 | 0.17±0.00 | 90 |
Rel2= % drug released after 2 hr;
Rel24 = % drug released after 24 hr;
Rel120 = % drug released after 120 hr;
DE = dissolution efficiency;
MDT=mean dissolution times;
Flux= amount of drug permeated per unit surface area versus time,
Papp= Apparent permeability
Drug release kinetics studies
Among the kinetic models employed, the Weibull’s distribution model exhibited the best fit compared to the other ones, with an R2 of 0.994 and an MPE% of 5.36%.
Ex vivo permeation studies
The present study also evaluated the ex vivo permeation of DEX from the two different drug forms (micelles and marketed eye drop). The obtained data are represented in Table 3. Micelles showed a cumulative (%) permeation of 24.33% of DEX in 24 hr, but the permeation was 20.96% for the marketed drop (Figure 12). The DEX micelles had a nearly two times higher apparent permeability (0.33×10-6 cm/sec) compared to the marketed drop (0.17×10-6 cm/sec) (P<0.05).
Figure 12.

Cumulative amount of drug penetrated through the unit surface area of the bovine corneal membrane in unit of time from the micelles and marketed drop
Histopathological evaluation studies
Histological inspection of the cornea sections stained by hematoxylin/eosin dye was performed to detect any possible damage to the cornea tissue upon the use of the prepared formulations (Figure 13). These studies demonstrated no evidence of detectable damage to the integrity of the cornea.
Figure 13.

Histopathological evaluation of the bovine cornea sections, untreated (A), treated with micelles (B) (magnitude X)
Discussion
Solubility is one of the important physicochemical properties of pharmaceutical compounds. Various approaches have been used to enhance the solubility of poorly water-soluble drugs, including particle size reduction, solid dispersion, nanosuspension, supercritical fluid technology, cryogenic technology, inclusion complex formation techniques (32), salt formation, use of surfactants (33), etc. The objective of this study was to enhance the solubility and prolong the precorneal residual time of DEX using the PCL-PEG-PCL copolymers. For this purpose, the tri-block copolymer of the PCL-PEG-PCL was synthesized via the ring-opening copolymerization. FT-IR and 1H-NMR results indicated the successful synthesis of the copolymer.
The crystalline nature of the samples was investigated using XRD and DSC. The peaks appeared in the XRD diffractogram of the copolymer showed that the copolymer had a crystalline nature. The reappearance of these peaks in the diffractogram of the dried film suggested that the copolymer recrystallized upon the evaporation of the organic phase. A noticeable decrease observed in the intensity of the drug peaks in the diffractogram of the physical mixture could have been due to the very small drug to polymer ratio (1:20). These characteristic peaks were not seen in the diffractogram of the dried film either. This observation could be either due to the molecular dispersion of the drug in the prepared film or the transition of the drug to the amorphous form.
The disappearance of the DEX peak in the thermogram of the dried film could be the result of either the change of the drug to an amorphous form or of the molecular dispersion of DEX in the prepared film. No peak related to the melting point of the drug was evident in the thermogram of the physical mixture, suggesting that the drug could also be dissolved in the melted polymer.
The crystallinity of PCL prevents the polymer to form micelle via spontaneous self-assembly. However, heating the polymer to its melting point gives PCL fragments the mobility needed and makes it possible for the polymer to self-assemble into the micelle (8). So the copolymer dispersions were heated to 60 °C, a temperature above the melting point of the copolymer according to the DSC data, followed by quenching in an ice bath to allow the copolymer to self-assemblies into micelles.
Rheological experiments were performed using a cone and plate viscometer. The viscosity of the copolymer solution increased upon exposure to eye temperature. Increasing the viscosity can lead to improved bioavailability because of both an enhanced pre-corneal retention time and a reduced nasolacrimal drainage of the drug.
The CMC of the PCL-PEG-PCL micelles was deter-mined using pyrene. Pyrene is poorly soluble in water. It is preferably solubilized in the hydrophobic core of micelles. The fluorescence of pyrene is sensitive to environmental change and the ratio of intensities of the first peak at 373 nm and the third one at 384 nm (II/IIII) can be used as the index of the polarity of its microenvironment. Below the CMC, the intensity values remain almost constant, but above this concentration, the ratio decreases considerably that indicates the incorporation of the probe into the interior hydrophobic region of the polymeric micelles. The ranges of observed variation of this ratio in a hydrocarbon solvent, a polar solvent, and a micellar medium are 0.57-0.61, 1.25-2, and 1.1-1.5, respectively (23).
The lower CMC values lead to an increment in the thermodynamic stability of the polymeric micelles (34). So, the obtained CMC (0.03 mg/ml) indicated moderate stability of the prepared PCL-PEG-PCL micelles against dilution.
The in vitro release of DEX from the micelles carried out in STF containing 0.5% Tween 80. According to the release date, the duration of DEX release from the micelles was longer (5 days) than that of the marketed eye drop (2 days). This could be attributed to the small porosity of the polymer matrix that served as an obstacle to drug diffusion.
The ex vivo permeation study of the DEX-loaded PCL-PEG-PCL micelles was performed in comparison with the marketed eye drop. The diffusion of substances through the ophthalmic barriers depends on various factors, including the chemical nature, lipid-water partition coefficient, size and conformation, and degree of ionization of the penetrative molecules tested (35, 36). The hydrophilic stroma, which constitutes 85-90% of the cornea, acts as a rate-limiting barrier for topically applied hydrophobic drugs (37). The observed enhancement in the permeability with micelles could be related to the encapsulating of hydrophobic DEX into the lipophilic core of highly water-soluble polymeric micelles. According to other studies (38, 39), these nanosized micelles can enhance the uptake of the micelle-DEX construct into corneal and conjunctival cells, and attributed to their hydrophilic corona, they can diffuse freely through the stroma. However, since the prepared micelles had a sustained release pattern, the observed difference between the permeability data was not remarkable, and the marketed drop showed even a higher permeation percent during the first hours of the study. Anyway, as mentioned earlier, the prepared formulation is expected to have an enhanced precorneal retention time due to an enhanced viscosity upon exposure to eye temperature. The nanosized micelles will then uptake into the cornea and release the loaded drug for a longer time. However, to prove these assumptions, an in vivo study is strongly desirable.
Conclusion
The PCL-PEG-PCL tri-block copolymer was successfully synthesized and assessed for its molecular weight, PDI, particle size, critical micelle concentration, and zeta potential. The film method was used to prepare drug-loaded micelles and achieve higher DEX solubility. The micelles were spherical in shape and had a narrow size distribution. The micelles were found to provide a low initial burst release followed by a sustained release, which best fitted the Weibull release kinetics. From a certain time on, the micelles showed greater transcorneal permeation compared to the marketed drop. Paracellular transport of the micelles across the sclera and the conjunctiva may cause in higher DEX levels in the intraocular tissues following topical administration. In addition, cellular entrance procedures such as endocytosis may be influenced by the size of the micelles. It may be the major mechanism for drug uptake into the corneal and conjunctival cells. As an outcome of corneal uptake of the micelles, the cornea functions like a reservoir and DEX can be released in a controlled manner. The long-term release of the drug would probably lead to an enhancement of treatment efficacy. Hence, the resulting micelles are promising for decreasing the dose frequency and fulfilling the patient compliance for ocular delivery.
Acknowledgment
The financial support from the Research Council of Tabriz University of Medical Sciences (under the grant No.111) is greatly acknowledged. The results described in this paper were part of a PhD student thesis registered at Tabriz University of Medical Sciences, Tabriz, Iran.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Agrawal RV, Murthy S, Sangwan V, Biswas J. Current approach in diagnosis and management of anterior uveitis. Indian J Ophthalmol. 2010;58:11–19. doi: 10.4103/0301-4738.58468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hogan MJ, Kimura SJ, Thygeson P. Signs and symptoms of uveitis. I. anterior uveitis. Am J Ophthalmol. 1959;47:155–170. doi: 10.1016/s0002-9394(14)78239-x. [DOI] [PubMed] [Google Scholar]
- 3.Gonjari ID, Karmarkar AB, Khade TS, Hosmani AH, Navale RB. Use of factorial design in formulation and evaluation of ophthalmic gels of gatifloxacin: comparison of different mucoadhesive polymers. Drug Discov Ther. 2010;4:423–434. [PubMed] [Google Scholar]
- 4.Gulsen D, Chauhan A. Ophthalmic drug delivery through contact lenses. Invest Ophthalmol Vis Sci. 2004;45:2342–2347. doi: 10.1167/iovs.03-0959. [DOI] [PubMed] [Google Scholar]
- 5.Le Bourlais C, Acar L, Zia H, Sado PA, Needham T, Leverge R. Ophthalmic drug delivery systems - recent advances. Prog Retin Eye Res. 1998;17:33–58. doi: 10.1016/s1350-9462(97)00002-5. [DOI] [PubMed] [Google Scholar]
- 6.Patel A, Cholkar K, Agrahari V, Mitra AK. Ocular drug delivery systems: an overview. World J Pharmacol. 2013;2:47–64. doi: 10.5497/wjp.v2.i2.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pepiæ I, Lovriæ J, Filipoviæ-Grèiæ J. Polymeric micelles in ocular drug delivery: rationale, strategies and challenges. CABEQ. 2012;26:365–377. [Google Scholar]
- 8.Vaishya RD, Gokulgandhi M, Patel S, Minocha M, Mitra AK. Novel dexamethasone-loaded nanomicelles for the intermediate and posterior segment uveitis. AAPS PharmSciTech. 2014;15:1238–1251. doi: 10.1208/s12249-014-0100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tiffany JM. Tears in health and disease. Eye. 2003;17:923–926. doi: 10.1038/sj.eye.6700566. [DOI] [PubMed] [Google Scholar]
- 10.Zhang W, Prausnitz MR, Edwards A. Model of transient drug diffusion across cornea. J Control Release. 2004;99:241–258. doi: 10.1016/j.jconrel.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Kopeček J, Kopečková P, Minko T, Lu ZR, Peterson CM. Water soluble polymers in tumor targeted delivery. J Control Release. 2001;74:147–158. doi: 10.1016/s0168-3659(01)00330-3. [DOI] [PubMed] [Google Scholar]
- 12.Vargas A, Pegaz B, Debefve E, Konan-Kouakou Y, Lange N, Ballini JP, et al. Improved photodynamic activity of porphyrin loaded into nanoparticles: an in vivo evaluation using chick embryos. Int J Pharm. 2004;286:131–145. doi: 10.1016/j.ijpharm.2004.07.029. [DOI] [PubMed] [Google Scholar]
- 13.Salatin S, Barar J, Barzegar-Jalali M, Adibkia K, Kiafar F, Jelvehgari M. Development of a nanoprecipitation method for the entrapment of a very water soluble drug into eudragit RL nanoparticles. Res Pharm Sci. 2017;12:1–14. doi: 10.4103/1735-5362.199041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ideta R, Tasaka F, Jang WD, Nishiyama N, Zhang GD, Harada A, et al. Nanotechnology-based photodynamic therapy for neovascular disease using a supramolecular nanocarrier loaded with a dendritic photosensitizer. Nano Lett. 2005;5:2426–2431. doi: 10.1021/nl051679d. [DOI] [PubMed] [Google Scholar]
- 15.Jang WD, Nakagishi Y, Nishiyama N, Kawauchi S, Morimoto Y, Kikuchi M, et al. Polyion complex micelles for photodynamic therapy: incorporation of dendritic photosensitizer excitable at long wavelength relevant to improved tissue-penetrating property. J Control Release. 2006;113:73–79. doi: 10.1016/j.jconrel.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 16.Takeuchi Y, Ichikawa K, Yonezawa S, Kurohane K, Koishi T, Nango M, et al. Intracellular target for photosensitization in cancer antiangiogenic photodynamic therapy mediated by polycation liposome. J Control Release. 2004;97:231–240. doi: 10.1016/j.jconrel.2004.03.030. [DOI] [PubMed] [Google Scholar]
- 17.Vaishya RD, Khurana V, Patel S, Mitra AK. Controlled ocular drug delivery with nanomicelles. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2014;6:422–437. doi: 10.1002/wnan.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trivedi R, Kompella UB. Nanomicellar formulations for sustained drug delivery: strategies and underlying principles. Nanomed Nanotech Biol Med. 2010;5:485–505. doi: 10.2217/nnm.10.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vadlapudi AD, Mitra AK. Nanomicelles: an emerging platform for drug delivery to the eye. Ther Deliv. 2013;4:1–3. doi: 10.4155/tde.12.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boddu SH, Jwala J, Vaishya R, Earla R, Karla PK, Pal D, et al. Novel nanoparticulate gel formulations of steroids for the treatment of macular edema. J Ocul Pharmacol Ther. 2010;26:37–48. doi: 10.1089/jop.2009.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gómez-Gaete C, Tsapis N, Besnard M, Bochot A, Fattal E. Encapsulation of dexamethasone into biodegradable polymeric nanoparticles. Int J Pharm. 2007;331:153–159. doi: 10.1016/j.ijpharm.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 22.Bae SJ, Suh JM, Sohn YS, Bae YH, Kim SW, Jeong B. Thermogelling poly(caprolactone-b-ethylene glycol-b-caprolactone) aqueous solutions. Macromolecules. 2005;38:5260–5265. [Google Scholar]
- 23.Ma G, Miao B, Song C. Thermosensitive PCL-PEG-PCL hydrogels: synthesis, characterization, and delivery of proteins. J Appl Polym Sci. 2010;116:1985–1993. [Google Scholar]
- 24.Zhou S, Deng X, Yang H. Biodegradable poly(ε-caprolactone)-poly(ethylene glycol) block copolymers: characterization and their use as drug carriers for a controlled delivery system. Biomaterials. 2003;24:3563–3570. doi: 10.1016/s0142-9612(03)00207-2. [DOI] [PubMed] [Google Scholar]
- 25.Liu CB, Gong CY, Huang MJ, Wang JW, Pan YF, Zhang YD, et al. Thermoreversible gel-sol behavior of biodegradable PCL-PEG-PCL triblock copolymer in aqueous solutions. J Biomed Mater Res B Appl Biomater. 2008;84:165–175. doi: 10.1002/jbm.b.30858. [DOI] [PubMed] [Google Scholar]
- 26.Alkhalidi BA, Alkhatib HS, Khdair AA. Comparative dissolution of diltiazem immediate and extended release products using conventional USP and innovative dissolution paddles. TODDJ. 2010;4:48–54. [Google Scholar]
- 27.Elsayed I, Abdelbary AA, Elshafeey AH. Nanosizing of a poorly soluble drug: technique optimization, factorial analysis, and pharmacokinetic study in healthy human volunteers. Int J Nanomedicine. 2014;9:2943–2953. doi: 10.2147/IJN.S63395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mpharm KD, Ramana MV, Sara UV, Agrawal DK, Mpharm KP, Chakravarthi S. Preparation and evaluation of transdermal plasters containing norfloxacin: a novel treatment for burn wound healing. Eplasty. 2010;10:e44. [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen THA, Nguyen VC. Formation of nanoparticles in aqueous solution from poly(σ- caprolactone)- poly(ethylene glycol)-poly(σ-caprolactone) Adv Nat Sci: Nanosci Nanotechnol. 2010:1. [Google Scholar]
- 30.Barghi L, Asgari D, Barar J, Valizadeh H. Synthesis of PCEC copolymers with controlled molecular weight using full factorial methodology. Adv Pharm Bull. 2015;5:51–56. doi: 10.5681/apb.2015.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tamboli V, Mishra GP, Mitra AK. Novel pentablock copolymer (PLA-PCL-PEG-PCL-PLA)-based nanoparticles for controlled drug delivery: effect of copolymer compositions on the crystallinity of copolymers and in vitro drug release profile from nanoparticles. Colloid Polym Sci. 2013;291:1235–1245. doi: 10.1007/s00396-012-2854-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Savla S, Surjusee A, Rokade V, Sawant S, Kadu P. Approaches to improve solubility of poorly water soluble drugs. Word J Pharm Pharmaceut Sci. 2015;4:610–626. [Google Scholar]
- 33.Sareen S, Mathew G, Joseph L. Improvement in solubility of poor water-soluble drugs by solid dispersion. Int J Pharm Investig. 2012;2:12–17. doi: 10.4103/2230-973X.96921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaihara S, Fisher JP, Matsumura S. Chemo-enzymatic synthesis of degradable PTMC-b-PECA-b-PTMC triblock copolymers and their micelle formation for pH-dependent controlled release. Macromol Biosci. 2009;9:613–621. doi: 10.1002/mabi.200800308. [DOI] [PubMed] [Google Scholar]
- 35.Pignatello R, Bucolo C, Spedalieri G, Maltese A, Puglisi G. Flurbiprofen-loaded acrylate polymer nanosuspensions for ophthalmic application. Biomaterials. 2002;23:3247–3255. doi: 10.1016/s0142-9612(02)00080-7. [DOI] [PubMed] [Google Scholar]
- 36.Shegokar R, Müller RH. Nanocrystals: industrially feasible multifunctional formulation technology for poorly soluble actives. Int J Pharm. 2010;399:129–139. doi: 10.1016/j.ijpharm.2010.07.044. [DOI] [PubMed] [Google Scholar]
- 37.Lee VHL, Robinson JR. Topical ocular drug delivery: recent developments and future challenges. J Ocul Pharmacol. 1986;2:67–108. doi: 10.1089/jop.1986.2.67. [DOI] [PubMed] [Google Scholar]
- 38.Aksungur P, Demirbilek M, Denkbaş EB, Vandervoort J, Ludwig A, Ünlü N. Development and characterization of cyclosporine A loaded nanoparticles for ocular drug delivery: cellular toxicity, uptake, and kinetic studies. J Control Release. 2011;151:286–294. doi: 10.1016/j.jconrel.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 39.Mandal A, Bisht R, Rupenthal ID, Mitra AK. Polymeric micelles for ocular drug delivery: from structural frameworks to recent preclinical studies. J Control Release. 2017;248:96–116. doi: 10.1016/j.jconrel.2017.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]


