Fig. 3.
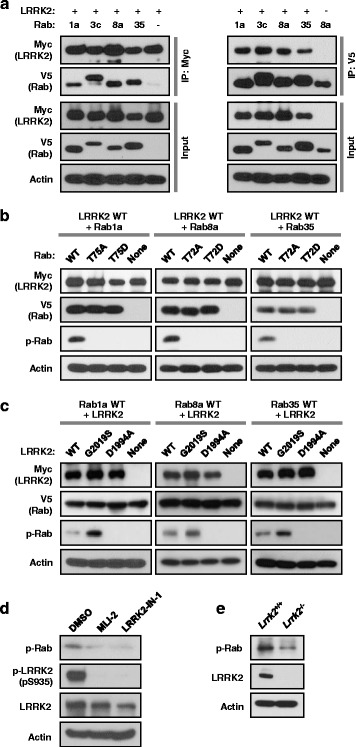
LRRK2 and Rab interaction and Rab phosphorylation by LRRK2 in cells. a Co-immunoprecipitation of LRRK2 and Rab proteins. Human embryo kidney (HEK)-293 cells were co-transfected with myc-tagged LRRK and V5-tagged Rab1a, 3c, 8a, or 35, and immunoprecipitated using anti-myc or V5 antibodies, followed by western blot analysis. (b-c) Verification of Rab phosphorylation by LRRK2 in HEK-293 cells. Expression and phosphorylation of Rab and/or LRRK2 were monitored by western blot analysis, and actin level was monitored as a loading control. Rab phosphorylation was examined by phospho-site-specific Rab antibodies (p-Rab) generated against a phosphopeptide corresponding to the LRRK2 site in Rab1a, as described in Methods. For b, cells were transfected either with LRRK2 wild-type (WT) alone (depicted as “None” in each set) or along with WT or phosphomutants (TA or TD) of Rab1a, 8a, or 35. For c, cells were transfected either with Rab1a, 8a, or 35 (all WTs) alone (depicted as “None” in each set) or along with WT or mutants (G2019S or D1994A) of LRRK2. d NIH-3T3 cells were treated with MLi-2 (1 μM), LRRK2-IN-1 (1 μM), or vehicle control, and phosphorylation of endogenous Rab and endogenous LRRK2 was monitored by western blot analysis using anti-p-Rab and anti-pLRRK2 (phospho-serine 935) antibodies. e Phosphorylation of endogenous Rab in WT and Lrrk2 knockout mice. Brain lysates from WT and Lrrk2 knockout mice were subjected to western blot analysis with the indicated antibodies. All results shown are representative blots from three independent experiments
