Abstract
Background:
Unilateral ureteral obstruction (UUO) alters the expression of renin-angiotensin system (RAS) components and angiotensin 1-7 (Ang 1-7) as a main arm of RAS is affected by UUO. The role of Mas receptor antagonist (A779) was examined in renal hemodynamic responses to Ang 1-7 in 3-day UUO and UUO removal (RUUO) in rats.
Materials and Methods:
Forty-five male Wistar rats were randomly divided into three groups of sham operated, UUO, and RUUO, while each group was divided into two subgroups treated with vehicle or A779. Renal blood flow (RBF) and renal vascular resistance (RVR) responses to graded Ang 1-7 infusion were measured at controlled renal perfusion pressure.
Results:
Mean arterial pressure response to Ang 1-7 was increased in vehicle-treated subgroup significantly (P < 0.05) when compared with A779-treated subgroup. However, such observation was not seen in UUO and RUUO rats. The graded Ang 1-7 infusion increased RBF and decreased RVR significantly in vehicle-treated rats (P < 0.005). Furthermore, a significant difference was found between vehicle and A779-treated subgroups in sham, UUO, and RUUO groups (P < 0.005).
Conclusion:
Ang 1-7 could alter the kidney hemodynamics responses in ureteral obstruction models.
Keywords: A779, Angiotensin 1-7, Mas receptor, renal blood flow, renal vascular resistance, unilateral ureteral obstruction
Introduction
Unilateral ureteral obstruction (UUO) is caused due to occlusion of ureters, which leads to renal hemodynamic impairment and injury.[1] UUO alters the production of local vasoactive factors, increases renal vascular resistance (RVR), and decreases the renal blood flow (RBF) in ipsilateral kidney before UUO removal (RUUO).[2] The therapeutic strategies of UUO have remained as main goal of urologists,[3] and renin-angiotensin system (RAS) is one of the main systems which plays an important role in the regulation of the hemodynamic parameters and tubular effects in UUO model.[4] Angiotensin 1-7 (Ang 1-7) via Mas receptor (MasR) as a main arm of RAS exhibits the vasodilatory properties against angiotensin II (Ang II).[5] Ang 1-7 via MasR has vasodilatory, antiproliferative, antidiuretic, and antinatriuretic properties in the kidney.[6,7] In addition, angiotensin-converting enzyme 2 (ACE2)/Ang 1-7/MasR axis has renoprotective effects and used as a therapeutic agent in kidney diseases.[8] Activation of ACE2/Ang 1-7/MasR axis decreases the reactive oxygen species accumulation in kidney tissue and inhibits the fibrosis in experimental glomerulonephritis.[9,10] It has been shown that MasR expression altered in UUO model and genetic deletion of MasR leads to fibrosis in ipsilateral kidney suffering from UUO.[11,12] Moreover, although the role of Ang II was studied in UUO model,[13] there is lack of information related to Ang 1-7. Therefore, this study was designed to assess the role of MasR antagonist (A779) in renal hemodynamic responses to graded Ang 1-7 infusion in rats with 3-day UUO and 24 h after RUUO.
Materials and Methods
Animal
Male Wistar rats (215 ± 15 g) were obtained from Water and Electrolyte Research Center Animal House, Isfahan University of Medical Sciences. The rats were housed at 23°C–25°C with a 12-h light/dark cycle and allowed 1 week to acclimatize this situation. The rats had free access to rat chow and tap water ad libitum. This experimental protocol was approved in advance by the Isfahan University of Medical Sciences Ethics Committee (Ethic number: IR. MUI. REC.1395.3.353) and all experiments were executed in accordance with the guidelines for Animal Care and Use of Laboratory Animals (National Institutes of Health Publication No. 85-23) revised 2010. Animals were randomly divided into three groups of sham operated, UUO, and RUUO, and each group was subdivided into two subgroups (n = 8–10 per subgroup) treated with vehicle or A779 as following (the medications dosage is indicated in the experimental protocol):
Sham group: Animals were subjected to operation without ureteral obstruction. Three days later, they were divided into two subgroups treated with vehicle (saline) and A779, then the each of subgroup followed by the Ang 1-7 infusion.
UUO group: Animals were subjected to operation with UUO. Three days later, they were divided into two subgroups treated with vehicle and A779, then the each of subgroup followed by the Ang 1-7 infusion.
RUUO group: Animals were subjected to operation with UUO. Three days later, the obstruction was removed, and they were divided into 2 subgroups treated with vehicle and A779, then the each of subgroup followed by the Ang 1-7 infusion.
Experimental surgery
Induction of unilateral ureteral obstruction and unilateral ureteral obstruction removal models
Animals were anesthetized with chloral hydrate (450 mg/kg, I.P injection, Sigma St. Louis, USA) and through an incision on the left quadrant of abdomen, UUO induced with ligated of the left ureter with 4-0 nylon suture, and the incision was closed in two layers by 4-0 silk suture. In sham groups, the left ureter was similarly exposed and manipulated without the ureter occlusion. Rectal temperature was maintained at 37°C ± 1°C using a heated lamp during the entire period of the surgical procedure. UUO or sham procedures continued 3 days after surgery. In RUUO groups, UUO was removed after 3 days. 24 h later, 3 groups were entered into the surgical preparation that has been mentioned.
Surgical preparation
The rats were anesthetized with urethane (1.7 g/kg, I.P injection, Sigma St. Louis, USA) and trachea was cannulated for suitable ventilation. The left jugular vein was cannulated by polyethylene catheters (PE 9658, Microtube Extrusions, North Rocks NSW, Australia) for the vehicle (saline) or A779 and Ang 1-7 infusion. In addition, catheters were inserted into the left carotid and femoral arteries and then were connected to a pressure transducer and a bridge amplifier (Scientific Concepts, Vic., Melbourne, Australia) for measuring the mean arterial pressure (MAP) and renal perfusion pressure (RPP), respectively. Furthermore, the bladder was catheterized to facilitate urine output. Then, the left kidney was exposed and placed in a stable kidney cup. Renal artery was isolated, and then an ultrasound flow meter probe (T108; Transonic Systems) was positioned around the renal artery for RBF measuring continuously throughout the experiment. RVR was obtained by dividing RPP to RBF. An adjustable clamp was placed around the abdominal aorta above the left renal artery to the regulation of RPP in base levels during Ang 1-7 infusion. Rat's body temperature was monitored via rectal thermometer (Model HB101/2; AgnTho's AB, Lidingo, Sweden) throughout the experiment and maintained at 36.5°C –37.5°C.[14,15]
Experimental protocol
Response to antagonist
Baseline measurements for the MAP, RPP, and RBF were recorded as equilibrium phase over the 30–45-min period. The animals with abnormal baseline data were excluded from the study. Then, each subgroup based on the sham or antagonist was subjected to receive vehicle (saline) or MasR blocker, A779 (Bachem, King of Prussia, MO, USA). A779 dissolved in 0.9% w/v saline was administered as bolus doses of 50 μg/kg followed to the end of the study by continuous infusion of 50 μg/kg/h using a microsyringe pump (New Era Pump System Inc., Farmingdale, NY, USA).[16] MAP, RPP, and RBF have measured 30 min post vehicle/antagonist infusion and were considered as MAP, RPP, and RBF responses to the antagonist.
Response to Ang 1-7 infusion
The graded Ang 1-7 (30, 100, 300, and 1000 ng/kg/min) was infused intravenously via another microsyringe pump 30 min after A779 or vehicle was started to infuse each dose of Ang 1-7 was infused for a 15 min period while RPP was kept at pre-Ang 1-7 administration levels with aortic clamp manipulation. MAP, RPP, and RBF responses to graded Ang 1-7 were determined over the final 3–5 min of each infusion. Ang 1-7 doses are based on other studies.[14] At the end of experiment, the rats were sacrificed humanely via high doses of the anesthetic drug. RVR was calculated by RPP/RBF ratio.
Statistical analysis
The data were expressed as mean ± standard error of the mean and analysis was performed using SPSS V20 software (SPSS Inc., Chicago). The baseline data and the effect of vehicle/antagonist were analyzed by the one-way ANOVA. Inter- and between-group comparisons were followed by least significant difference (LSD) test. A repeated-measure ANOVA was applied to compare the effect of each treatment responses to Ang 1-7. Significant differences were considered with values of P ≤ 0.05.
Results
Baseline measurements
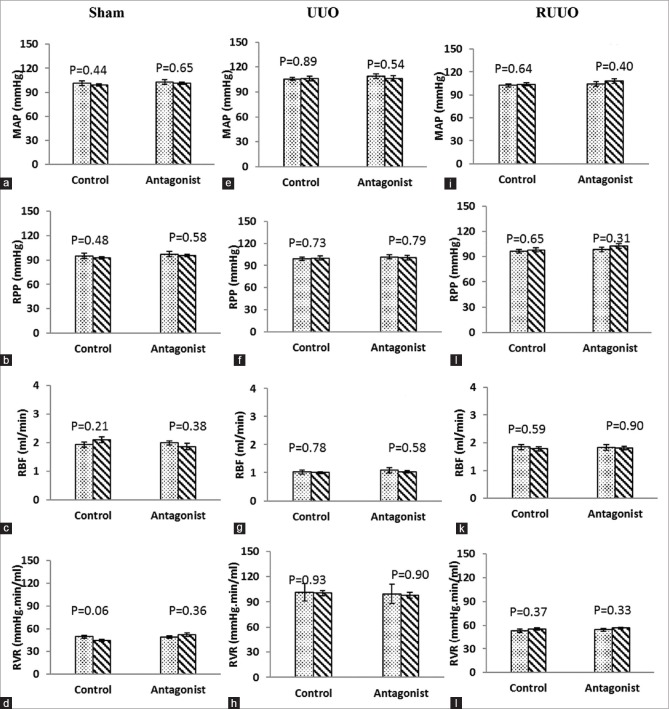
The baseline data (in equilibrium period) for two subgroups of vehicle or A779 were not significantly different in terms of MAP, RPP, RBF, and RVR in groups of sham, UUO, and RUUO [Figure 1a–l].
Figure 1.
(a-l) The hemodynamic parameters before and after vehicle or A779 infusion in the rat (Sham, UUO, or RUUO models). Data are shown as mean ± standard error of the mean and P values were derived from one-way ANOVA (LSD test comparisons). UUO: Unilateral ureteral obstruction, RUUO: UUO removal, MAP: Mean arterial pressure, RPP: Renal perfusion pressure, RBF: Renal blood flow, RVR: Renal vascular resistance
Effect of vehicle/antagonist
The results indicated that vehicle or A779 infusion had no significant effect on MAP, RPP, RBF, and RVR in groups of sham, UUO, and RUUO [Figure 1a–l].
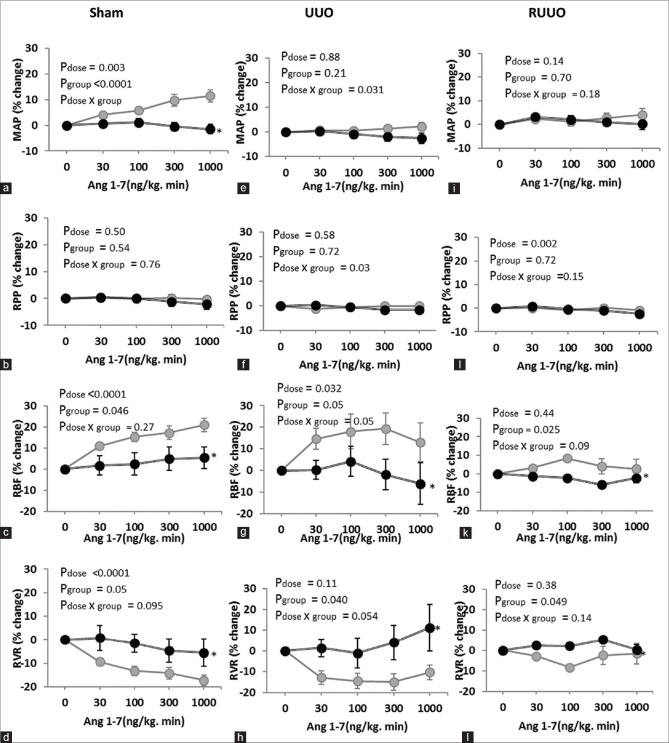
Response to graded Ang 1-7 infusion
In the sham group, the graded Ang 1-7 infusion increased MAP and RBF and decreased RVR in vehicle-treated subgroups significantly when compared with A779-treated subgroups (P < 0.005). In addition, in UUO and RUUO groups, MAP responses to Ang 1-7 were not altered significantly [Figure 2a–l]. However, RBF responses to Ang 1-7 were significantly increased and the alterations were different between vehicle and A779 subgroups, for example, 300 ng/kg/min Ang 1-7 infusion increased the RBF percentage changes to 17.28% ± 3.23, 19.38% ± 7.34 and 4.05 ± 4.16, respectively, in sham, UUO, and RUUO rats treated with saline while this percentage changes were 4.94% ± 5.44, −1.89% ± 7.12 and −5.86% ± 1.13 in A779-treated groups. Moreover, RVR responses to Ang 1-7 was significantly decreased in vehicle-treated rats compared with A779-treated rats in groups of sham, UUO, and RUUO [Figure 2d, h, and l].
Figure 2.
(a-l) The percentage changes of the MAP, RPP, RBF, and RVR in vehicle or A779 administration rats’ responses to graded Ang 1-7 infusion in sham or UUO or RUUO models. Data are presented as mean ± standard error of the mean of percentage changes from baseline. The P values were derived from repeated-measure ANOVA. ∗: Represents significant difference from the vehicle. UUO: Unilateral ureteral obstruction, RUUO: UUO removal, MAP: Mean arterial pressure, RPP: Renal perfusion pressure, RBF: Renal blood flow, RVR: Renal vascular resistance
Discussion
The main findings of this study indicated that the graded infusion of Ang 1-7 significantly altered the RBF and RVR responses in sham, UUO, and RUUO groups when MasR was not blocked. It has been made clear that Ang 1-7 via MasR significantly improves the renal injury with suppressing the fibrosis and apoptosis in the kidney with UUO.[17] In our study, 3-day UUO significantly decreased RBF [Figure 1c and g] and increased RVR [Figure 1d and h] in ipsilateral kidney but did not have a significantly effect on the MAP. In agreement with our study, it has been shown that hydronephrosis in one kidney did not have significant effect on blood pressure[18] and also UUO did not alter systolic blood pressure significantly.[18] A779 decreases the RBF in intact rats[19] and exacerbates the renal fibrosis,[20] and genetic deletion of MasR induces renal fibrosis.[21] In the current study, A779 infusion alone could not significantly alter the MAP and hemodynamic parameters in each of sham, UUO, and RUUO rats [Figure 1a–l]. Similar to our study, it is reported that A779 did not alter systolic blood pressure and plasma parameters in UUO model.[20] In addition, our results showed that Ang 1-7 increases the RBF and decreases the RVR in sham, UUO, and RUUO rats when MasR was not blocked. Moreover, the RBF response to Ang 1-7 was different in vehicle-treated rats rather than A779-treated group in sham, UUO, and RUUO groups [Figure 2c–l]. Ren et al.[22] showed that Ang 1-7 via MasR induced its dilatation effects on afferent arterioles in rabbits. Also, in our study, it seemed that the graded Ang 1-7 infusion improved the renal hemodynamic indicators in kidney suffering UUO. In this regard, Zhang et al.[10] reported that Ang 1-7 attenuates the glomerulosclerosis and reduces the proteinuria in glomerulonephritis rats. Also, it has been noted that endogenous Ang 1-7 via MasR exerts renoprotective effects,[11] and infusion of Ang 1-7 reduces renal injury in the kidney with UUO.[20] Hence, it seems that the hemodynamic response to A779 did not differ significantly in A779 groups with each other, but this is significantly different when compared with sham-operated groups. Moreover, it has been shown that angiotensin receptor 1 expression increased in obstructed kidney[12] and Ang 1-7 can interact with the other receptors.[23] Furthermore, some of its vasodilator effects possibly obtained from anatomization of renal vasoconstrictor effects of Ang II.[24] Since in our study, no response was detected by MasR blocked in each group of sham, UUO, and RUUO, probably, the block of MasR disrupts the situation in the same way in all sham, UUO, and RUUO conditions. This condition has even been observed in other diseases, so it has been reported that Ang 1-7 decreases inflammation and renal injury via MasR pathways.[11] In another part of our results, we showed that RVR and RBF responses to graded Ang 1-7 infusion were highlighted in UUO compared with RUUO subgroups and this different response can be generated through vascular resistance [Figure 2g, k, h, and l]. Since short-term UUO can continue with progressive kidney injury after RUUO,[25] this factor can also be effective for the different response to Ang 1-7 in RUUO rats.
Conclusion
The graded Ang 1-7 infusion could alter the renal hemodynamic response in all sham, UUO, and RUUO groups treated with saline that possibly generated via MasR interaction because Ang 1-7 could increase RBF in each model when MasR is not blocked. According to our study, Ang 1-7 may be useful as a treatment therapist following UUO or RUUO conditions in the future as its renoprotective effects in hemorrhagic shock were reported.[26]
Financial support and sponsorship
This research was supported by Isfahan University of Medical Sciences (Grant # 395353).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Vaughan ED, Jr, Marion D, Poppas DP, Felsen D. Pathophysiology of unilateral ureteral obstruction: Studies from charlottesville to New York. J Urol. 2004;172:2563–9. doi: 10.1097/01.ju.0000144286.53562.95. [DOI] [PubMed] [Google Scholar]
- 2.Tavukçu HH, Tinay İ, Tarcan T. To save or not to save the kidney: Relieving unilateral obstruction may significantly improve an initially low split renal creatinine clearance. J Urol Surg. 2016;3:7–12. [Google Scholar]
- 3.Gulmi FA, Felsen D. Smith's Textbook of Endourology. Vol. 12. West Sussex (United Kingdom): Wiley-Blackwell; 2012. Pathophysiology of urinary tract obstruction; pp. 95–119. [Google Scholar]
- 4.Hvistendahl JJ, Pedersen TS, Djurhuus JC, Pedersen EB, Frøkiaer J. Losartan attenuates renal vasoconstriction in response to acute unilateral ureteral occlusion in pigs. Urol Res. 2002;30:169–77. doi: 10.1007/s00240-002-0240-y. [DOI] [PubMed] [Google Scholar]
- 5.Santos RA, Ferreira AJ. Angiotensin-(1-7) and the renin-angiotensin system. Curr Opin Nephrol Hypertens. 2007;16:122–8. doi: 10.1097/MNH.0b013e328031f362. [DOI] [PubMed] [Google Scholar]
- 6.Santos RA, Simoes e Silva AC, Maric C, Silva DM, Machado RP, de Buhr I, et al. Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor mas. Proc Natl Acad Sci U S A. 2003;100:8258–63. doi: 10.1073/pnas.1432869100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinheiro SV, Simões e Silva AC, Sampaio WO, de Paula RD, Mendes EP, Bontempo ED, et al. Nonpeptide AVE 0991 is an angiotensin-(1-7) receptor mas agonist in the mouse kidney. Hypertension. 2004;44:490–6. doi: 10.1161/01.HYP.0000141438.64887.42. [DOI] [PubMed] [Google Scholar]
- 8.Zimmerman D, Burns KD. Angiotensin-(1-7) in kidney disease: A review of the controversies. Clin Sci (Lond) 2012;123:333–46. doi: 10.1042/CS20120111. [DOI] [PubMed] [Google Scholar]
- 9.Jawien J, Toton-Zuranska J, Gajda M, Niepsuj A, Gebska A, Kus K, et al. Angiotensin-(1-7) receptor Mas agonist ameliorates progress of atherosclerosis in apoE-knockout mice. Atherosclerosis. 2012;1:4. [PubMed] [Google Scholar]
- 10.Zhang J, Noble NA, Border WA, Huang Y. Infusion of angiotensin-(1-7) reduces glomerulosclerosis through counteracting angiotensin II in experimental glomerulonephritis. Am J Physiol Renal Physiol. 2010;298:F579–88. doi: 10.1152/ajprenal.00548.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zimmerman DL, Zimpelmann J, Xiao F, Gutsol A, Touyz R, Burns KD, et al. The effect of angiotensin-(1-7) in mouse unilateral ureteral obstruction. Am J Pathol. 2015;185:729–40. doi: 10.1016/j.ajpath.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 12.Esteban V, Heringer-Walther S, Sterner-Kock A, de Bruin R, van den Engel S, Wang Y, et al. Angiotensin-(1-7) and the g protein-coupled receptor MAS are key players in renal inflammation. PLoS One. 2009;4:e5406. doi: 10.1371/journal.pone.0005406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Radović N, Aralica G, Ljubanović DG, Jelec V, Kontek M. Effect of unilateral ureteral obstruction and anti-angiotensin II treatment on renal tubule cell apoptosis and interstitial fibrosis in rats. Coll Antropol. 2014;38:583–8. [PubMed] [Google Scholar]
- 14.Nematbakhsh M, Safari T. Role of mas receptor in renal blood flow response to angiotensin (1-7) in male and female rats. Gen Physiol Biophys. 2014;33:365–72. doi: 10.4149/gpb_2014008. [DOI] [PubMed] [Google Scholar]
- 15.Saberi S, Dehghani A, Nematbakhsh M. Role of mas receptor in renal blood flow response to angiotensin-(1-7) in ovariectomized estradiol treated rats. Res Pharm Sci. 2016;11:65–72. [PMC free article] [PubMed] [Google Scholar]
- 16.Mansoori A, Oryan S, Nematbakhsh M. Role of mas receptor antagonist (A779) on pressure diuresis and natriuresis and renal blood flow in the absence of angiotensin II receptors type 1 and 2 in female and male rats. J Physiol Pharmacol. 2014;65:633–9. [PubMed] [Google Scholar]
- 17.Kim CS, Kim IJ, Bae EH, Ma SK, Lee J, Kim SW, et al. Angiotensin-(1-7) attenuates kidney injury due to obstructive nephropathy in rats. PLoS One. 2015;10:e0142664. doi: 10.1371/journal.pone.0142664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arnold AC, Shaltout HA, Gilliam-Davis S, Kock ND, Diz DI. Autonomic control of the heart is altered in sprague-dawley rats with spontaneous hydronephrosis. Am J Physiol Heart Circ Physiol. 2011;300:H2206–13. doi: 10.1152/ajpheart.01263.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sampaio WO, Nascimento AA, Santos RA. Systemic and regional hemodynamic effects of angiotensin-(1-7) in rats. Am J Physiol Heart Circ Physiol. 2003;284:H1985–94. doi: 10.1152/ajpheart.01145.2002. [DOI] [PubMed] [Google Scholar]
- 20.Zimmerman D. The Role of Angiotensin-(1-7) in a Mouse Model of Renal Fibrosis. University of Ottawa (Canada); 2013. [Google Scholar]
- 21.Pinheiro SV, Ferreira AJ, Kitten GT, da Silveira KD, da Silva DA, Santos SH, et al. Genetic deletion of the angiotensin-(1-7) receptor mas leads to glomerular hyperfiltration and microalbuminuria. Kidney Int. 2009;75:1184–93. doi: 10.1038/ki.2009.61. [DOI] [PubMed] [Google Scholar]
- 22.Ren Y, Garvin JL, Carretero OA. Vasodilator action of angiotensin-(1-7) on isolated rabbit afferent arterioles. Hypertension. 2002;39:799–802. doi: 10.1161/hy0302.104673. [DOI] [PubMed] [Google Scholar]
- 23.Bosnyak S, Jones ES, Christopoulos A, Aguilar MI, Thomas WG, Widdop RE, et al. Relative affinity of angiotensin peptides and novel ligands at AT1 and AT2 receptors. Clin Sci (Lond) 2011;121:297–303. doi: 10.1042/CS20110036. [DOI] [PubMed] [Google Scholar]
- 24.Stegbauer J, Vonend O, Oberhauser V, Rump LC. Effects of angiotensin-(1-7) and other bioactive components of the renin-angiotensin system on vascular resistance and noradrenaline release in rat kidney. J Hypertens. 2003;21:1391–9. doi: 10.1097/00004872-200307000-00030. [DOI] [PubMed] [Google Scholar]
- 25.Ito K, Chen J, El Chaar M, Stern JM, Seshan SV, Khodadadian JJ, et al. Renal damage progresses despite improvement of renal function after relief of unilateral ureteral obstruction in adult rats. Am J Physiol Renal Physiol. 2004;287:F1283–93. doi: 10.1152/ajprenal.00441.2003. [DOI] [PubMed] [Google Scholar]
- 26.Maleki M, Nematbakhsh M. Renal blood flow response to angiotensin 1-7 versus hypertonic sodium chloride 7.5% administration after acute hemorrhagic shock in rats. Int J Vasc Med 2016. 2016:6562017. doi: 10.1155/2016/6562017. [DOI] [PMC free article] [PubMed] [Google Scholar]