Abstract
Cell therapy is an emerging paradigm for the treatment of heart disease. In spite of the exciting and promising preclinical results, the benefits of cell therapy for cardiac repair in patients have been modest at best. Biomaterials-based approaches may overcome the barriers of poor differentiation and retention of transplanted cells. In this study, we prepared and tested hydrogels presenting extracellular matrix (ECM)-derived adhesion peptides as delivery vehicles for c-kit+ cardiac progenitor cells (CPCs). We assessed their effects on cell behavior in vitro as well as cardiac repair in rats undergoing ischemia reperfusion. Hydrogels presenting the collagen-derived GFOGER peptide induced cardiomyocyte differentiation of CPCs as demonstrated by increased expression of cardiomyocyte structural proteins. However, conditioned media obtained from GFOGER hydrogels showed lower levels of secreted reparative factors. Interestingly, following injection in rats undergoing ischemia-reperfusion, treatment with CPCs encapsulated in nonadhesive RDG-presenting hydrogels resulted in the preservation of cardiac contractility and attenuation of postinfarct remodeling whereas the adhesion peptide-presenting hydrogels did not induce any functional improvement. Retention of cells was significantly higher when delivered with nonadhesive hydrogels compared to ECM-derived peptide gels. These data suggest that factors including cell differentiation state, paracrine factors and interaction with biomaterials influence the effectiveness of biomaterials-based cell therapy. A holistic consideration of these multiple variables should be included in cell-biomaterial combination therapy designs.
Keywords: stem cell, biomaterials, hydrogel, integrin, cardiac progenitor cell
Graphical Abstract
INTRODUCTION
Cardiovascular disease is the leading global cause of death. In the United States, cardiovascular diseases are responsible for 1 in every 4 deaths and pose an economic burden of over $200 billion per year.1 Myocardial ischemia results in large-scale cardiomyocyte death by necrosis and apoptosis, which commences a cascade of compensatory remodeling mechanisms.2 The pathological remodeling following cellular loss is a common cause for heart failure. Existing treatment strategies, such as pharmacological agents, lifestyle modification, and reperfusion, slow down the progression of remodeling but do not reverse or halt it. Development of heart failure is linked to high morbidity and mortality and can only be cured by heart transplantation.3
Cell therapy is an emerging treatment strategy for cardiac repair. C-kit+ cardiac progenitor cells (CPCs) are adult stem cells that have been shown to differentiate into cardiomyocytes, endothelial cells and vascular smooth muscle cells, and exert beneficial paracrine effects.4–6 They are safe for delivery as determined in phase I clinical trials and do not pose ethical issues. These cells can be easily isolated and expanded, and then delivered autologously or allogeneically to patients.7,8 These attributes make CPCs an attractive choice for cell therapy for heart failure and other cardiovascular diseases. Despite the positive outlook and exciting preclinical study results, injection of CPCs in humans in phase I trials resulted in moderate but likely insufficient improvements in cardiac function after myocardial infarction.7 This suggests the need for enhancements to cell function to maximize their reparative potential.
The poor outcome of cell therapy is generally attributed to the rapid wash out of injected cells as well as the hostility of the environment in the infarcted myocardium.9 Biomaterials have the potential of overcoming these obstacles by allowing retention of cells and providing them a custom microenvironment in the infarcted myocardium.10,11 Maleimide-cross-linked poly(ethylene glycol) (PEG-MAL) hydrogels are attractive vehicles for cell delivery to the heart as they maintain high cell viability and are injectable. Their high tunability in terms of their mechanical properties, degradation rate, and ability to present sites for linkage of bioactive ligands for cell adhesion and stimulation also makes them attractive for translation.12 Previous studies demonstrate that these versatile hydrogels can deliver therapeutic proteins to the myocardium following infarction in rats.13
Extracellular matrix (ECM) proteins present sites for cell adhesion and influence cell behavior including induction of differentiation, proliferation and enhancement of paracrine effects. For example, CPCs cultured on collagen show increased connexin43 expression, suggesting potential cardiomyogenic differentiation.14 Culturing CPCs on fibronectin leads to increased proliferation and survival under stress.15 Therefore, natural and synthetic biomaterials presenting ECM-inspired ligands can be used as cell carriers to enhance cell therapy effects. ECM-derived adhesion peptides can be covalently conjugated to PEG-maleimide through Michael-type addition reactions. We hypothesized that ECM-derived peptide presenting hydrogels will enhance CPC-based therapy by activating signaling involved in reparative processes.
CPCs were encapsulated in hydrogels presenting a RGD peptide derived from various ECM proteins including fibronectin, the triple helical, collagen-mimetic peptide GFOGER, or nonadhesive scrambled peptide RDG. Basal integrin expression on CPCs and rheological properties of hydrogels were characterized. In vitro expression of lineage markers to assess differentiation of CPCs and secreted factors following encapsulation in ECM-derived peptide gels or nonadhesive control gels were measured. In vivo effects of delivery of these constructs on cardiac function in an ischemia-reperfusion model and ex vivo analyses of cardiac fibrosis, angiogenesis, hypertrophy, and retention of exogenous cells were performed.
MATERIALS & METHODS
CPC Isolation and Culture
C-kit+ cardiac progenitor cells (CPCs) were isolated from human atrial tissue obtained from children aged 1 week or less undergoing corrective surgery at Children’s Healthcare of Atlanta, following Institutional Review Board approval as previously published.16 The tissue was rinsed with cold Hank’s Buffered Salt Solution (HBSS) and chopped into small pieces. Enzymatic degradation of ECM was performed by incubation with 1 mg/mL collagenase II (Worthington) solution at 37 °C, 5% CO2 for 30 min and the solution was then passed through a 70 μm strainer (BD). Magnetic beads (Dynal) conjugated to anti-c-kit antibodies (Santa Cruz H-300) were prepared. The cells were mixed with beads conjugated to anti-c-kit antibody and incubated on a rocker in a humidified cell culture incubator for 2 h. Magnetic beads were incubated with digested tissue to bind c-kit+ cells and then separated using a magnet, interspersed with two washes. The separated c-kit+ cells were expanded and expression of c-kit in the collected population was measured by flow cytometry to ensure they were at least 90% positive. Cells from three donors were pooled at the first passage and used for our experiments. Human CPC (hCPC) cell culture media included Ham’s F-12 base media (Corning), 100 U/mL penicillin–0.1 mg/mL streptomyocin cocktail (Cellgro), 2 mM L-glutamine (Cellgro), 0.1 μg/mL basic fibroblast growth factor (Sigma) and 10% heat inactivated fetal bovine serum (Hyclone). Media was refreshed every 2–3 days.
Hydrogel Synthesis and Cell Encapsulation
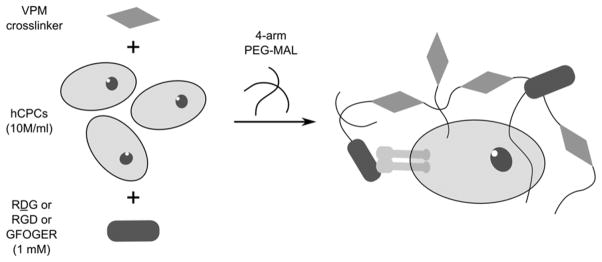
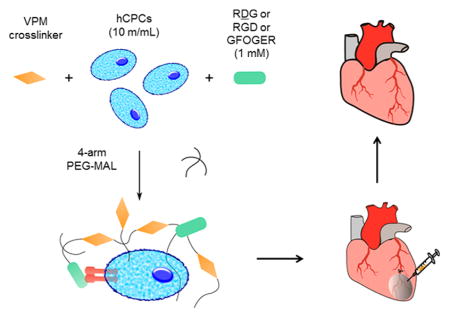
Poly(ethylene glycol)-based hydrogels encapsulating cells and different peptides were prepared using a modified one-step functionalization procedure. Components were resuspended in pH 6.0 10 mM HEPES in Dulbecco’s PBS with Ca2+ and Mg2+ (DPBS++). A premixed solution of the protease-sensitive cross-linker peptide “VPM” (GCRDVPMS-MRGGDRCG) (New England Peptide), hCPCs (10 million cells/mL hydrogel), and nonadhesive ligand ‘RDG' (GRDGSPC) (AAPPTec) or “RGD” (GRGDSPC) (New England Peptide) or “GFOGER” (GGYGGGP(GPP)5GFOGER(GPP)5GPC) (AAPPTec) was mixed with 20 kDa PEG-MAL (Laysan Bio). The final concentration of the RDG/RGD/GFOGER peptide was 1.0 mM, 4% or 5% w/v for PEG and the concentration of VPM (New England Peptide) was equal to the balance maleimide sites remaining after accounting for maleimides theoretically reacting with the 1.0 mM adhesive/scrambled ligands. A schematic of the hydrogel synthesis procedure is shown in Figure 1. The solutions were mixed in a 1 mL syringe barrel as a mold and allowed to gel at 37 °C for 10 min, transferred to a 24 well plate containing 500 μL/well Ham’s F-12 media (without serum) with 1x Insulin-Transferrin-Selenium (Cellgro), 100 U/mL penicillin–0.1 mg/mL streptomyocin cocktail (Cellgro), and 2 mM L-glutamine (Cellgro) (CPC treatment media). Media was changed every 2–3 days.
Figure 1.
Schematic of hydrogel synthesis. A solution of VPM cross-linker, 10 million/mL cell suspension and adhesive (RGD, GFOGER) or nonadhesive (RDG) ligands was prepared. The gels were cross-linked by mixing with 4-arm PEG-MAL macromer solution. Final concentrations of components in hydrogel solution were 1.0 mM of RDG/RGD/GFOGER, 5% w/v of PEG-MAL and VPM cross-linker concentration was calculated by determining balance maleimides after accounting for other ligands.
Integrin Expression and Cell Adhesion
mRNA analysis was performed by real-time PCR. A standard curve was prepared using human atrial tissue to obtain mRNA copy number and GAPDH was used as a housekeeping gene. Protein expression was measured using colorimetric α/β integrin mediated cell adhesion array (EMD Millipore ECM532), following the manufacturer’s protocol. Briefly, cells were dislodged from tissue culture flasks using Versene (Life Technologies) and integrin surface expression measured using ELISA array plates read using a plate reader (Biotek Synergy2). For measuring cell adhesion, cell-free hydrogels presenting RDG or RGD and 2 mg/mL collagen gels (Cellgro) were prepared in 96-well plates and swollen in DPBS++ for 2 h. CPCs were washed and incubated with 3 μM Calcein for 20 min, dislodged (TrypLE) and plated on gels (50k cells/well) in serum and phenol free DPBS++. After 20 min, cells were washed using DPBS++ and fluorescence measured using a plate reader (Biotek Synergy2).
Rheology
As described in a previous publication,17 cell-free hydrogels were made, swollen in DPBS++ overnight and their storage and loss moduli measured using dynamic oscillatory strain and frequency sweeps performed on a MCR 302 stress-controlled rheometer (Anton Paar, Austria) with a 9 mm diameter, 28 cone, and plate geometry. The hydrogels were loaded between the cone and plate, and the measuring system was lowered to a 39 μm gap. Initial strain amplitude sweeps were performed at ω = 10 rad/s to determine the linear viscoelastic range of the hydrogel. Oscillatory frequency sweeps (0.5–100 rad/s) were then used to determine the storage and loss moduli at a strain of 1%.
Real-Time PCR
Cells were harvested in Trizol (Life Technologies) and total RNA was isolated by following the manufacturer’s protocol. For isolating RNA from cells encapsulated in hydrogels, the hydrogels were homogenized in Trizol using a homogenizer (Fisher Scientific PowerGen 500). RNA quantification and purity were determined by absorbance readings at 260 and 280 nm (BioTek Synergy2 Spectrophotometer). cDNA was prepared from mRNA using a MuMLV reverse transcriptase-based reaction. Real-time polymerase chain reaction was performed and analyzed using Step One Software (Applied Biosystems). ΔΔCt method was used to obtain fold change values over the specified control. Primer sequences are provided in Table S1.
Western Blotting for Cardiac Proteins
For analyzing cells encapsulated in hydrogels, the hydrogels were degraded by incubation with 1 mg/mL collagenase I (Worthington) solution for 40 min and the released cells were pelleted by centrifugation. Cells were homogenized in NP-40 lysis buffer supplemented with protease inhibitor (Sigma-Aldrich P2714) and phosphatase inhibitor cocktails (Cell Signaling Technology 5870S). The lysate was centrifuged at 10 000g for 10 min. The supernatant was stored at –80 °C until further analysis and the pellet was resuspended in RIPA buffer. Equal total protein amount samples, as measured by micro BCA, were loaded on SDS-PAGE gels. The separated proteins were transferred onto nitrocellulose membranes, blocked using 5% bovine serum albumin dissolved in tris-buffered saline with 0.1% Tween-20 (BSA-TBST) at RT for 1 h, incubated with primary antibody at 4 °C overnight, washed with TBST three times for 10 min each, incubated with 1:5000 v/v horseradish peroxidase-conjugated secondary antibody (Bio-Rad) at RT for 1 h, washed three times for 10 min each, incubated with the substrate ECL (Denville Scientific) or ECL Prime (GE) and then exposed to an X-ray film (Denville Scientific). The films were scanned at 600 dpi resolution and obtained bands were quantified by densitometry analysis in ImageJ (NIH). Information on antibodies used is provided in Table S2.
Conditioned Media Immunoassay
Conditioned media from cell-laden hydrogels was separated, centrifuged at 10 000g for 10 min to remove particulate matter, and supernatant stored at –80 °C until further analysis. Dilutions (1:2) of the samples were processed using a Luminex kit (R&D Systems- LXSAH) following the manufacturer’s protocol. Briefly, the samples were added to a mixture of color-coded beads that were precoated with analyte-specific capture antibodies. Biotinylated detection antibodies specific to the analytes of interest were added to from an antibody–antigen sandwich. Phycoerythrin (PE)-conjugated streptavidin was added to bind biotinylated detection antibodies. The beads were analyzed on a Luminex 100 instrument. Concentrations were obtained from fluorescence readings by mapping on to a standard curve.
Animal Studies
All animal experiments were performed with the approval of the Institutional Animal Care and Use Committee of Emory University. Athymic rats (Crl:NIH-Foxn1rnu) (~250 g, 6–8 weeks old) were obtained from Charles River Laboratory. Rats were anesthetized with 2% isoflurane, orally intubated, and ventilated. The left anterior descending coronary artery was ligated for 30 min followed by reperfusion. During reperfusion, 60 μL of hydrogels were injected into the myocardium at three border zone locations using a 275/8G insulin syringe (BD) similar to a previous report.13 Cardiac function was evaluated 7 and 28 d after surgery by echocardiography (Acuson Sequoia 512 with a 14 MHz transducer). All functional evaluations were conducted and analyzed by investigators blinded to the animal treatment group. Ejection fraction, fractional shortening and left ventricular internal diameter measurements were obtained by analyzing scans in M-mode (Vevo 2100). The rats were euthanized 4 weeks after surgery and their hearts were excised and processed for histological analyses. Sham group rats underwent opening of chest under anesthesia but their artery was neither ligated nor reperfused. IR only animals underwent artery ligation and reperfusion but did not receive any injection.
Histology and Immunostaining
Explanted hearts were fixed in 4% paraformaldehyde at 4 °C overnight or RT for 4 h, dehydrated in ethanol and embedded in paraffin using Leica TP1020 tissue processor. Five to seven micrometer thick sections were made. The paraffin-embedded heart tissue sections were dewaxed in Histoclear (National Diagnostics) followed by a series of washes in ethanol.
Picrosirius Red
The sections were stained with picrosirius red solution for 1 h, washed in acidified water and ethanol, and mounted with resinous medium (Cytoseal). Whole slide scans were taken (Hamamatsu) and the percentage of fibrosis was quantified from low resolution images using Aperio software as the ratio of fibrotic tissue (stained red) to total tissue.
Isolectin
The sections underwent antigen retrieval in pH 6 citrate buffer for 10 min and were incubated with FITC-tagged isolectin (Vector) for 1 h at RT or 4 °C overnight. Three randomly chosen sections in the infarct border region were photographed and the number of fluorescent cells also positive for DAPI were manually counted.
Wheat Germ Agglutinin
The sections underwent antigen retrieval in pH 6 citrate buffer for 10 min and incubated with rhodamine-tagged isolectin (Vector) for 30 min at RT. The cross-sectional areas of 6–8 myocytes in the infarct border region were measured by manual tracing (CellSens software).
Human Mitochondria
The sections underwent antigen retrieval in pH 6 citrate buffer for 20 min, permeabilization with 0.1% Triton X for 10 min and were incubated with antihuman mitochondria antibody (1:50 v/v, Abcam) overnight at 4 °C, followed by incubation with alkaline phosphatase-conjugated secondary antibody (1:50 v/v, Sigma). The sections were incubated with Vector Red (Vector) substrate for 10 min, counterstained with methyl green, dehydrated in ethanol and mounted with resinous medium (Cytoseal). Whole slide scans were taken (Hamamatsu) and cells positive for both human mitochondria (pink color developed by alkaline phosphatase-Vector Red reaction) and nuclei (green stain by methyl green) were counted (NDP view software).
All histological evaluations were performed by blinded investigators.
Statistics
All data are expressed as mean ± SEM. To determine significance of difference between means, either a one-way or two-way analysis of variance (ANOVA) followed by the appropriate posthoc test, or Student’s t test was performed using GraphPad Prism5, as specified with each result. A value of p < 0.05 was considered statistically significant.
RESULTS
Integrin Expression in hCPCs and Cell Adhesion to ECM-Derived Peptide Hydrogels
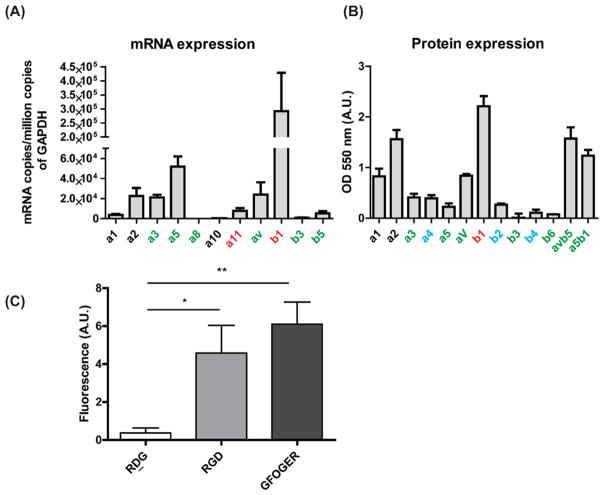
To assess the availability of integrin receptors as first points of contact with ECM-derived peptide-presenting hydrogels, mRNA expression of relevant integrins and protein expression on surface of hCPCs were measured. GFOGER is known to interact with α1β1, α2β1, α10β1 (found primarily in cartilage) and α11β1. RGD is a ligand for αvβ1, αvβ3, αvβ6, αvβ5, α5β1, α8β1, α11β3, and α3β1 integrins.18 A pool of hCPCs from three neonatal patients at passages ranging from P3–10 was tested. mRNA expression of integrin subunits was measured using real-time PCR and is shown in Figure 2A. Protein expression was measured using an ELISA array based on colorimetric detection and the results are shown in Figure 2B. Absolute values of integrins expressed on surface could not be measured because of lack of a standard curve. CPCs show prominent expression of β1 at both mRNA and surface protein level. They also express α2 and αv integrin subunits relevant to GFOGER and RGD ligands, respectively. The expression level of β3 was below detection level in assay of both mRNA and surface protein. Since αvβ3 is the major player in cell adhesion to RGD and α5β1 plays a minor role,19 cell adhesion to RGD gels was tested using a wash assay. As shown in Figure 2C, cells showed higher adhesion to RGD and GFOGER gels than nonadhesive RDG gels.
Figure 2.
hCPC integrin expression profile and adhesion. (A) mRNA expression determined by real-time PCR (n = 4–7) and (B) protein expression measured by ELISA in hCPCs (n = 4) of integrin subunits involved in adhesion to RGD (green) and GFOGER (black) molecules. Red letters indicate binding to both, and blue letters are binding to neither. (C) Adhesion to RDG- RGD-, and GFOGER-presenting hydrogels measured using cell binding assay (n = 8, ANOVA and Tukey’s posthoc test, *p < 0.05, **p < 0.01).
Mechanical Characterization of Hydrogels
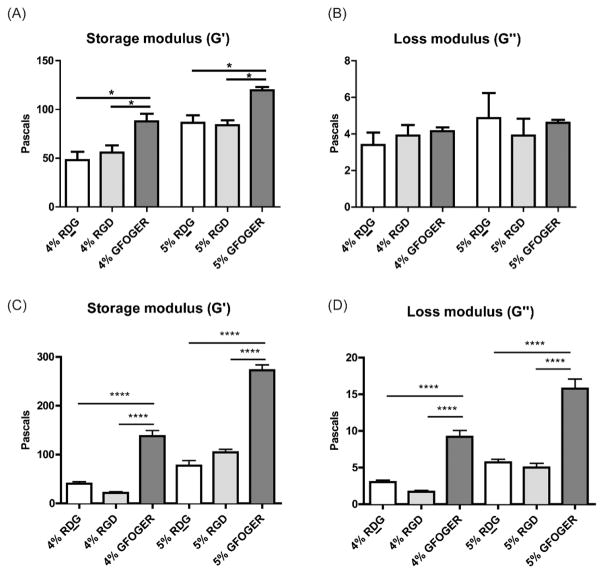
Mechanical properties of biomaterials are known to influence stem cell differentiation20 and therefore the mechanical properties of these hydrogels could impact the fate of transplanted cells. In addition, biomaterials are thought to dissipate wall stresses in infarcted hearts for which their mechanical properties are relevant as well.21 Rheological characterization of hydrogels was performed to measure their mechanical properties and results are shown in Figure 3. The storage moduli (G′) of RDG and RGD gels were similar but both were significantly lower than GFOGER gels on comparison of density-matched hydrogels. Loss moduli (G″) were found to be comparable among all groups.
Figure 3.
Rheological characterization. Storage moduli and loss moduli of (A and B) empty PEG-MAL hydrogels or (C and D) cellular PEG-MAL hydrogels of 4% or 5% w/v densities presenting 1.0 mM RDG, RGD or GFOGER peptides measured using rheology. n = 6–8, ANOVA and Tukey’s posthoc test,*p < 0.05; ****p < 0.0001.
Expression of Lineage Markers in Encapsulated CPCs
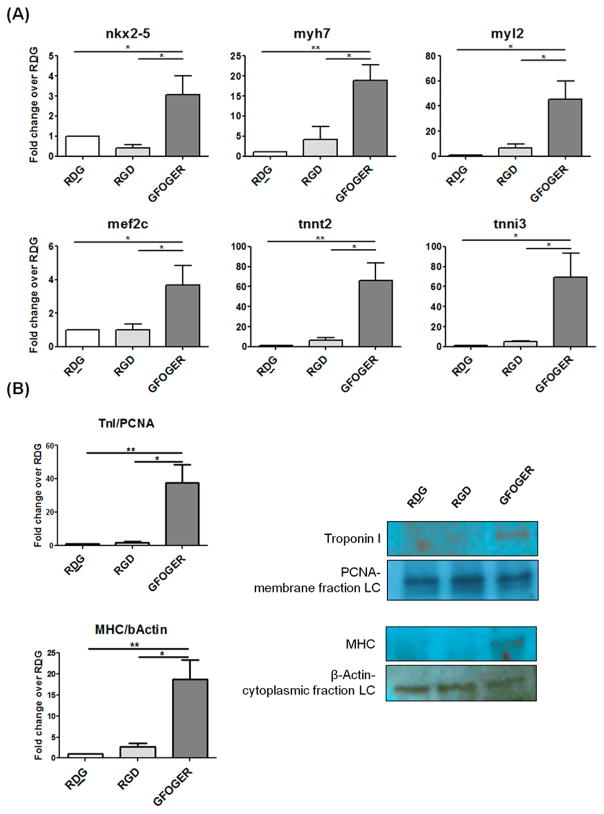
To assess the ability of ECM-derived peptide presenting gels to induce differentiation of CPCs via integrin signaling activation, mRNA expression of several lineage markers for cardiomyocyte, endothelial and vascular smooth muscle was performed. As shown in Figure 4A, mRNA expression of cardiac transcription factors nkx2_5 and mef 2c, cardiomyocyte specific structural proteins myh7, myl2, tnnt2, and ctnni3 were upregulated in cells encapsulated in GFOGER gels as measured by real-time PCR 2 d after encapsulation. mRNA expression of endothelial and vascular smooth muscle markers was not significantly different between the groups (Figure S1). To determine if this carried through at the protein level, expression of selected cardiomyocyte markers was measured. Protein levels of cardiomyocyte structural proteins myosin heavy chain (MHC) and troponin I (TnI) were significantly increased in GFOGER gel-encapsulated CPCs (Figure 4B) as measured by Western blotting at 2 d after embedding cells.
Figure 4.
Expression of lineage markers in encapsulated CPCs. (A) mRNA expression of cardiomyocyte lineage markers 2 d after encapsulation: transcription factors nkx2.5 and mef2c, and structural proteins: myh7, myl2, tnnt2, and tnni3. (B) Protein expression of Myosin heavy chain (normalized to β-actin) and Troponin I (normalized to proliferating cell nuclear antigen) measured using Western blotting and densitometry. Data are presented as fold change over respective RDG group. LC: Loading control. n = 3–4, ANOVA and Tukey’s posthoc test, *p < 0.05, **p < 0.001.
Paracrine Factors Released in Conditioned Media
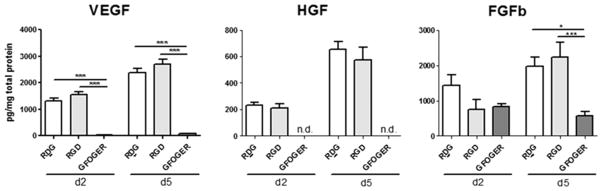
Paracrine factors have been shown repeatedly to be a very important mode by which stem cells including CPCs exert their effects.22 The modulation of paracrine factors by ECM-derived peptide presenting hydrogels was tested by measuring concentrations of several analytes in conditioned media obtained after 2 and 5 d. As shown in Figure 5, the levels of paracrine factors VEGF, HGF, and FGFb were significantly lower in the conditioned media of cell-laden GFOGER gels compared to conditioned media from RDG- and RGD-functionalized hydrogels at both d 2 and 5. RDG and RGD peptides had similar levels of these factors. IGF-1, MMP9, IL10, PDGF-BB, and SDF1 concentrations were below the range of detection and concentration of MMP2 was over the detection range.
Figure 5.
Secreted factors in conditioned media. Concentration of vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), and basic fibroblast growth factor (FGFb) in conditioned media at d2 and d5 measured using a Luminex bead-based multiplex assay. Obtained individual protein concentrations were normalized for sample loading by dividing by total protein in conditioned media. n = 4–8, ANOVA and Tukey’s posthoc test, *: p < 0.05, **: p < 0.001.
Cardiac Function
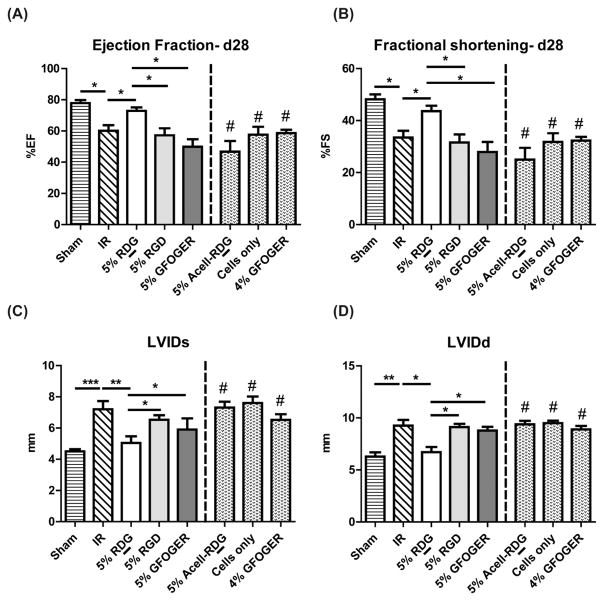
To determine if there were functional changes by cells encapsulated within hydrogels, we injected the indicated formulation immediately following ischemia-reperfusion (IR) injury. Results for ejection fraction (EF) and fractional shortening (FS) at 28 d after surgery are shown in Figure 6A. IR predictably resulted in a significant decrease in cardiac function compared to sham-operated animals. Interestingly, only animals that received cell therapy in RDG-presenting hydrogels significantly improved EF and FS values as compared to IR alone. Both RGD- and GFOGER-functionalized gel treatments did not show improvements in cardiac function as compared to IR animals. t d 28, cell-laden RDG gel group EF (72.21 ± 2.26%) and FS (43.03 ± 2.10%) values were significantly higher than IR group (EF: 63.30 ± 2.84, FS: 34.38 ± 2.40%) (p < 0.05). RGD (EF: 50.65 ± 7.76, FS: 27.10 ± 4.85%) and GFOGER (EF: 49.91 ± 4.82, FS: 27.91 ± 3.88%) groups showed inferior function and their EF and FS values were significantly lower than those of sham animals (EF: 77.92 ± 1.9, FS: 48.19 ± 1.86%). We performed additional controls in order to assess the contribution of components of the cell-laden RDG gels, that is, cell-free RDG gels and cells only groups; however, neither improved function when delivered individually. Additionally, as 4% GFOGER gels had comparable mechanical properties as the 5% RDG gels as measured by rheology, we also examined whether that treatment improved function. Similar to other controls, cells within 4% GFOGER did not improve function. Results for EF and FS at d 7 are shown in Figure S2.
Figure 6.
Cardiac function following treatment with ECM-derived peptide presenting hydrogels. Ejection fraction (A), fractional shortening (B), and left ventricular internal diameter at systole (LVIDs, C) and diastole (LVIDd, D) obtained using M-mode echocardiograms of rat hearts 28 d following treatment. n ≥ 3 for 4% GFOGER, Acell-RDG and n ≥ 5 for other groups, ANOVA followed by Bonferroni post-test comparisons with selected groups, *p < 0.05; **p < 0.01; p < 0.001; #p < 0.05 vs 5% RDG.
Histological Evaluation
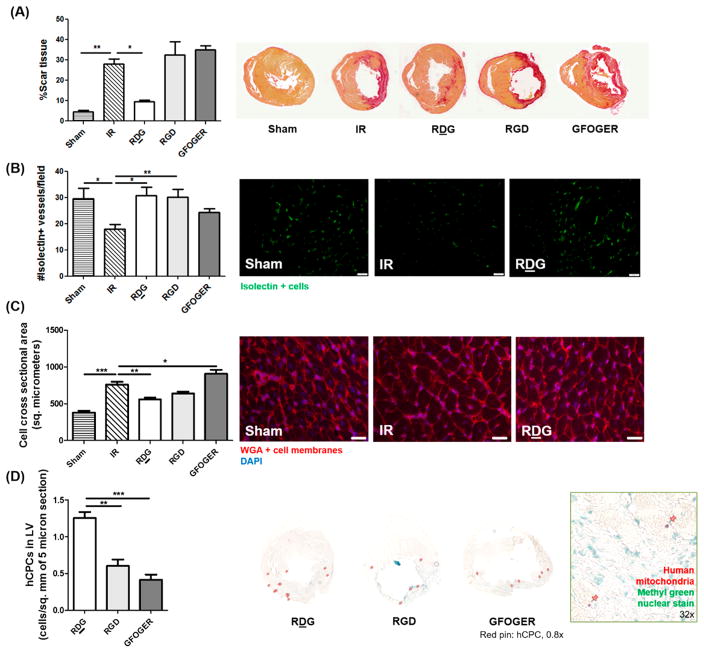
To gain insights on the observed cardiac performance following various treatments, histological evaluation of fibrosis, angiogenesis, hypertrophy, and retention of transplanted human cells was performed. As shown in Figure 7A, the percentage of scar tissue in cell-laden RDG gel group was significantly lower than IR only, with no effect of any other treatment. Angiogenesis was measured by staining sections with isolectin, which stains rodent endothelial cells and other cells expressing terminal α-galactosyl residues. Both RDG and RGD groups showed higher number of isolectin-positive vessels than IR only group at d 28, but the GFOGER group was not significantly different (Figure 7B). To determine hypertrophy of cardiomyocytes, cross-sectional areas were measured by tracing cell membranes stained by fluorescently tagged wheat-germ agglutinin. Figure 7C demonstrates that only in RDG treated animals was cross-sectional area of myocytes significantly improved compared to IR alone. Retention of transplanted cells at d 28 was measured by counting cells stained with human specific antimitochondria antibody in the left ventricular wall. Figure 7D shows that significantly higher number of human CPCs was found in sections from RDG treated animal hearts as compared to RGD and GFOGER gel treated hearts. The retention of transplanted cells correlated with improvement in function and reduction in scar tissue.
Figure 7.
Histological evaluation of rat hearts following treatment with hydrogels. Formalin fixed paraffin embedded hearts from rats sacrifficed 28 d following treatment were stained. (A) Fibrosis as measured by calculating percentage of scar tissue (red area) following picosiruius red staining, (B) angiogenesis measured by quantifying isolectin-positive vessels per field in the infarct border region (scale bar: 50 μm), (C) hypertrophy measured by quantifying cardiomyocyte cross-sectional area in infarct border region (scale bar: 20 μm), and (D) transplanted hCPC retention measured by quantifying human mitochondria positive (pink) cells in left ventricle (LV) of 5 μm sections. n = 3–6, ANOVA and Tukey’s posthoc test, *p < 0.05, **p < 0.001, ***p < 0.0001.
DISCUSSION
Cell therapy is undergoing clinical trials and numerous preclinical and clinical studies are being pursued to enhance the efficacy of cell therapy for treating heart failure. Biomaterials-based strategies have shown potential to improve outcomes of cell therapy because of many properties. These include the ability of biomaterials to enhance retention of cells, act as reserves of growth factors that can be presented to the cells, and create a custom microenvironment that promotes regeneration instead of the hostile environment in the heart post-MI.10 In this study, we evaluated encapsulated human pediatric-derived c-kit+ cardiac progenitor cells in PEG hydrogels presenting ECM-derived adhesion peptides: fibronectin-derived RGD, collagen-mimetic GFOGER, or control scrambled nonadhesive RDG peptide. GFOGER gels induced cardiomyocyte differentiation of CPCs and lowered levels of reparative secretive factors in vitro. In an in vivo model of ischemia reperfusion, only nonadhesive RDG gels codelivered with CPCs improved function whereas ECM-derived adhesive peptide-presenting gels did not.
Expression of integrins relevant to RGD and GFOGER ligands adhesion were measured at the mRNA level by real- time PCR and at protein level using ELISA and β1 was found to be the most abundant subunit through both real-time PCR and ELISA measurements, although the absolute concentration of protein could not be measured in the absence of a standard curve. β1 integrins are important participants in reparative mechanisms associated with cell therapy such as proliferation, migration, differentiation15,23,24 so the presence of this subunit in CPCs suggests that they may be good candidates for cardiac repair cell therapy. The expression of α2β1, which adheres to GFOGER with high affinity, was detected on the CPCs. β3, the major integrin involved in RGD binding was found to be below detection levels on our cells, keeping with prior studies on c-kit + cells obtained from the heart.25 Despite this, CPCs showed higher adhesion to RGD gels than nonadhesive RDG gels, though less than gels with GFOGER. The adhesion could be mediated by αvβ3 whose expression levels may be below detection limits of the assays used but sufficient to mediate adhesion. In the absence of αvβ3, other integrins such as α5β1, αvβ1 and αvβ5 may be playing a role in mediating adhesion of CPCs to RGD hydrogels.
As mechanical properties of hydrogels are important determinants of cell fate and in vivo performance, rheological characterization of 4% and 5% w/v hydrogels functionalized with RDG, RGD or GFOGER was performed with and without cells. The storage modulus was determined to be much greater than the loss modulus indicating that these gels are elastic in nature. GFOGER gels exhibited greater storage moduli than RDG and RGD gels of the same PEG density. While the same cross-linker and conditions were used for all the gels, it is possible that the longer GFOGER peptide is able to form a secondary structure that results in slightly stiffer gels. Despite its higher modulus, even the GFOGER hydrogel constructs were much softer than the heart.26 While native tissue-like stiffness of biomaterials is most conducive to differentiation,27–29 softer hydrogels are commonly used in studies perhaps because they are more likely to permit diffusion of nutrients and viability of encapsulated cells.30 Despite being softer than native heart tissue, we observed induction of cardiomyocyte differentiation of CPCs in only the GFOGER group. Along with biochemical factors, mechanical stimulation plays a significant role in inducing cardiomyocyte differentiation.20 Our observations of GFOGER-gel induced differentiation could be the result of the biological signal initiated by GFOGER, possibly mediated through α2β1 integrins, even in the absence of supplemental growth factors, and physical properties of the GFOGER-PEG hydrogel. Our finding that signaling initiated by collagen adhesive site GFOGER, perhaps through stimulation of α2β1 integrins, may be involved in CPC differentiation is novel. Other biochemical and physical cues such as aligned electrospun scaffolds, epigenetic modulators, growth factors, notch signaling modulators and stretch have been shown to induce cardiomyogenic differentiation of CPCs31–35 but the nature of biochemical cues driving the differentiation is not clear. Further investigation of α2β1 integrin stimulation as a method of cardiomyogenic differentiation induction should be performed using specific integrin blocking studies. It would also be interesting to determine the extent to which GFOGER stimulation can drive maturation of the cells by measuring expression of more mature markers like connexin43 and calcium channel proteins at this or a later time point.
Along with cardiomyocyte differentiation induction, we found significantly lowered expression of the paracrine factors HGF, VEGF and FGFb by cells in GFOGER gels as early as d 2 and d 5. RDG and RGD gels had similar levels of these growth factors in their conditioned media. HGF, VEGF, and FGFb show cardio-protective, mitogenic effects and direct stem cell homing to ischemic myocardium; VEGF and FGFb are angiogenic factors.36,37 In our study, differentiation of CPCs was accompanied by a lower release of beneficial paracrine factors. Such an observation has been made with c-kit+ BM-MSCs wherein 5-Aza treatment increased their cardiomyocyte differentiation but led to reduction in VEGF and bFGF expression by the cells. In this study, the less differentiated cells showed higher growth factor secretion and also led to the greatest cardiac recovery in vivo.38 The relation between paracrine factor levels and cardiomyocyte differentiation, however, is not always inverse suggesting the differentiation and paracrine factor release processes may be independent of each other or dependent on other factors. For example, culturing rat CPCs in Jagged-1-presenting hydrogels induced cardiomyocyte differentiation of the cells in vitro and in vivo implantation of this construct led to improvement in cardiac function post-MI. Conditioned media of the differentiating cells showed higher levels of SCF, CPC migration and proliferation and cardiomyocyte proliferation.34 In all of these cases though, as in our study, the groups with higher paracrine factor levels showed greater cardiac function improvement suggesting paracrine factors may be the main mechanism of cell therapy induced-benefits, even within biomaterials.
On injection of hydrogel-cell constructs in athymic rats that underwent ischemia-reperfusion, we found the nonadhesive RDG gel + CPCs combination to drive the greatest cardiac function improvement 4 weeks later. Gels presenting ECM-derived peptides RGD and GFOGER gels carrying CPCs failed to improve cardiac function which was unexpected as these ligands were expected to stimulate regenerative signaling in cardiac progenitor cells based on literature.14,15 Cell-free RDG gels did not increase cardiac function and neither did cells delivered without a hydrogel carrier (cells only group), suggesting the important role of both the RDG hydrogel carrier and cells in driving the improvement in EF and FS. Four percent GFOGER gels had comparable mechanical properties as 5% RDG gels but they also failed to improve cardiac function at d 28 suggesting that the mechanical properties were not solely responsible for the observed effects. These data point to the role of CPCs, PEG hydrogels and the nonadhesive ligands presented by the hydrogels in synergistically leading to cardiac function improvement of infarcted rat hearts. While we did not measure it in this study, it may be possible that the act of injection alone, or with saline, may induce an inflammatory reponse and this should be examined in future studies.
Given the cardiomyogenic differentiation of CPCs in GFOGER gels in vitro, we were expecting CPC + GFOGER gels to improve cardiac function more than the other combinations. This assessment was based on prior studies using CPCs that have shown differentiation inducing strategies to support greater cardiac function improvement.34,39 However, in those studies paracrine factors were upregulated along with differentiation or not measured. On the other hand, other studies examining different cell types have shown the less differentiated cells to demonstrate greater improvement in cardiac function owing to greater engraftment, paracrine factor release or survival under stress in less differentiated cells.38,40,41 We saw that GFOGER gels induced cardiomyogenic differ-entiation of CPCs and a reduction in paracrine factor levels in vitro. In vivo, GFOGER gels encapsulating CPCs could not preserve cardiac function and had less angiogenesis, and did not improve fibrosis or hypertrophy 4 weeks after treatment. The inability of GFOGER + CPC group to rescue cardiac function despite showing the greatest differentiation potential of all combinations studied points to the role of lowered paracrine effects observed in this group as a potential explanation.
The in vitro paracrine factor profile and differentiation state of CPCs, and dynamic modulus measured by rheology were comparable between RGD and RDG gel groups, so they cannot explain the differences between the two groups in vivo. At the 28 d time point, both RDG and RGD gels encapsulating CPCs showed significantly improved angiogenesis compared to IR only. Note that isolectin-positive cells were marked as surrogates for angiogenesis, which labels not just mature perfused large and micro vessels but endothelial cells of immature vessels as well.42 Retention of transplanted CPCs in left ventricle was significantly higher in RDG group than RGD group. In all groups, the number of human cells found in the rat heart at d 28 was much lower than what was injected, which is consistent with observations in other cell therapy studies and reinforces the paracrine hypothesis. Even though the number of retained transplanted cells was low, nonetheless, a correlation was found between the number of retained cells and improvement in cardiac function following cell delivery. Such a correlation between retention, even though low, and functional benefit has been observed with other cell types before.43–46
Other potential mechanistic explanations for our findings could be that the adhesive ligands may be inciting a stronger immune response leading to destruction and consequently lower retention of transplanted cells.47 Another possibility is that the ligands RGD and GFOGER may be blocking the integrins on delivered CPCs, even as the cells are released along with hydrogel degradation by VPM cleavage. This in turn may be preventing them from engaging with the infarcted myocardium through those integrins and possibly limiting their engraftment as has been seen with other cell types.44,48 In addition, the degradation of these hydrogels is protease-dependent and the protease activity in the environment of nonadhesive and adhesive hydrogels may be differential due to paracrine secretion. While prior studies show that the inclusion of binding peptides in PEG hydrogels does not alter degradation kinetics in vitro, we are unable at this time to measure these data in vivo. Degradation rate of nonadhesive gels may be better suited for engraftment of released cells into the injured host tissue and in turn improve cardiac function. Finally, future studies should investigate the use of CPC loaded nonadhesive hydrogels in clinically relevant immunocompetent rats and treatment administration at later time point equivalent to clinically practical scenarios as the immune response plays a significant role in post-MI pathophysiology and repair mechanisms.
CONCLUSIONS
In this paper, we tested ECM-derived peptide presenting hydrogels delivering CPCs as a therapeutic strategy for cardiac repair following myocardial infarction. In vitro findings showed hydrogels presenting GFOGER peptide mimicking collagen adhesive site to induce cardiomyogenic differentiation of CPCs accompanied by a reduction in release of reparative growth factors. However, contrary to expectations, only nonadhesive hydrogels showed improvement in cardiac function and prevention of maladaptive remodeling in rats that underwent ischemia-reperfusion. Overall, this study reveals novel insights about the behavior of CPCs in ECM-derived peptide presenting or nonadhesive scaffolds in vitro and response to these constructs in an animal model of ischemia-reperfusion. These findings add to our understanding of factors that influence the outcome of cell therapy and will help in future design of strategies for enhancing cell therapy for cardiac repair.
Supplementary Material
Acknowledgments
This work was supported by grant HL127236 to A.J.G. and M.E.D.
Footnotes
The authors declare no competing financial interest.
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsbiomater-ials.7b00502.
Tables for primers and antibodies and figures on acute cardiac function and endothelial marker gene expression (PDF)
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després J-P, Fullerton HJ. Heart Disease and Stroke Statistics—2016 Update. Circulation. 2016 doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Sutton MGSJ, Sharpe N. Left Ventricular Remodeling After Myocardial Infarction. Circulation. 2000;101(25):2981. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 3.Minicucci MF, Azevedo PS, Polegato BF, Paiva SAR, Zornoff LAM. Heart Failure After Myocardial Infarction: Clinical Implications and Treatment. Clin Cardiol. 2011;34(7):410–414. doi: 10.1002/clc.20922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miyamoto S, Kawaguchi N, Ellison GM, Matsuoka R, Shin’oka T, Kurosawa H. Characterization of Long-Term Cultured c-kit + Cardiac Stem Cells Derived From Adult Rat Hearts. Stem Cells Dev. 2010;19(1):105–116. doi: 10.1089/scd.2009.0041. [DOI] [PubMed] [Google Scholar]
- 5.Sharma S, Mishra R, Bigham GE, Wehman B, Khan MM, Xu H, Saha P, Goo YA, Datla SR, Chen L. A Deep Proteome Analysis Identifies the Complete Secretome as the Functional Unit of Human Cardiac Progenitor Cells. Circ Res. 2017;120:816. doi: 10.1161/CIRCRESAHA.116.309782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He J-Q, Vu DM, Hunt G, Chugh A, Bhatnagar A, Bolli A. Human Cardiac Stem Cells Isolated from Atrial Appendages Stably Express c-kit. PLoS One. 2011;6(11):e27719. doi: 10.1371/journal.pone.0027719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chugh AR, Beache GM, Loughran JH, Mewton N, Elmore JB, Kajstura J, Pappas P, Tatooles A, Stoddard MF, Lima JAC, et al. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: the SCIPIO trial: surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation. 2012;126:S54–64. doi: 10.1161/CIRCULATIONAHA.112.092627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lauden L, Boukouaci W, Borlado LR, Löpez IP, Sepúlveda P, Tamouza R, Charron D, Al-Daccak R. Allogenicity of Human Cardiac Stem/Progenitor Cells Orchestrated by Programmed Death Ligand 1Novelty and Significance. Circ Res. 2013;112(3):451–464. doi: 10.1161/CIRCRESAHA.112.276501. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen PK, Neofytou E, Rhee J-W, Wu JC. Potential Strategies to Address the Major Clinical Barriers Facing Stem Cell Regenerative Therapy for Cardiovascular Disease. JAMA Cardiol. 2016;1(8):953. doi: 10.1001/jamacardio.2016.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis ME, Hsieh PCH, Grodzinsky AJ, Lee RT. Custom design of the cardiac microenvironment with biomaterials. Circ Res. 2005;97(1):8–15. doi: 10.1161/01.RES.0000173376.39447.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Segers VFM, Lee RT. Biomaterials to enhance stem cell function in the heart. Circ Res. 2011;109(8):910–922. doi: 10.1161/CIRCRESAHA.111.249052. [DOI] [PubMed] [Google Scholar]
- 12.Phelps EA, Enemchukwu NO, Fiore VF, Sy JC, Murthy N, Sulchek TA, Barker TH, García AJ. Maleimide cross-linked bioactive PEG hydrogel exhibits improved reaction kinetics and cross-linking for cell encapsulation and in situ delivery. Adv Mater. 2012;24(1):64–70. doi: 10.1002/adma.201103574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salimath AS, Phelps EA, Boopathy AV, Che P, Brown M, García AJ, Davis ME. Dual delivery of hepatocyte and vascular endothelial growth factors via a protease-degradable hydrogel improves cardiac function in rats. PLoS One. 2012;7(11):e50980. doi: 10.1371/journal.pone.0050980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.French KM, Maxwell JT, Bhutani S, Ghosh-Choudhary S, Fierro MJ, Johnson TD, Christman KL, Taylor WR, Davis ME. Fibronectin and Cyclic Strain Improve Cardiac Progenitor Cell Regenerative Potential. Stem Cells Int. 2016;2016:1–11. doi: 10.1155/2016/8364382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Konstandin MH, Toko H, Gastelum GM, Quijada P, De La Torre A, Quintana M, Collins B, Din S, Avitabile D, Volkers M, et al. Fibronectin Is Essential for Reparative Cardiac Progenitor Cell Response After Myocardial Infarction. Circ Res. 2013;113(2):115–125. doi: 10.1161/CIRCRESAHA.113.301152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agarwal U, George A, Bhutani S, Ghosh-Choudhary S, Maxwell JT, Brown ME, Mehta Y, Platt MO, Liang Y, Sahoo S. Experimental, Systems and Computational Approaches to Understanding the MicroRNA-Mediated Reparative Potential of Cardiac Progenitor Cell-Derived Exosomes From Pediatric Patients. Circ Res. 2017;120:701. doi: 10.1161/CIRCRESAHA.116.309935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.García JR, Clark AY, García AJ. Integrin-specific hydrogels functionalized with VEGF for vascularization and bone regeneration of critical-size bone defects. J Biomed Mater Res, Part A. 2016;104(4):889–900. doi: 10.1002/jbm.a.35626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 19.Petrie TA, Capadona JR, Reyes CD, García AJ. Integrin specificity and enhanced cellular activities associated with surfaces presenting a recombinant fibronectin fragment compared to RGD supports. Biomaterials. 2006;27(31):5459–5470. doi: 10.1016/j.biomaterials.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 20.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 21.Wall ST, Walker JC, Healy KE, Ratcliffe MB, Guccione JM. Theoretical Impact of the Injection of Material Into the Myocardium: A Finite Element Model Simulation. Circulation. 2006;114(24):2627–2635. doi: 10.1161/CIRCULATIONAHA.106.657270. [DOI] [PubMed] [Google Scholar]
- 22.Sharma S, Mishra R, Bigham GE, Wehman B, Khan MM, Xu H, Saha P, Goo YA, Datla SR, Chen L. A Deep Proteome Analysis Identifies the Complete Secretome as the Functional Unit of Human Cardiac Progenitor Cells Novelty and Significance. Circ Res. 2017;120(5):816. doi: 10.1161/CIRCRESAHA.116.309782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie Y, Ibrahim A, Cheng K, Wu Z, Liang W, Malliaras K, Sun B, Liu W, Shen D, Cheol Cho H, et al. Importance of cell-cell contact in the therapeutic benefits of cardiosphere-derived cells. Stem Cells. 2014;32(9):2397–2406. doi: 10.1002/stem.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steingen C, Brenig F, Baumgartner L, Schmidt J, Schmidt A, Bloch W. Characterization of key mechanisms in transmigration and invasion of mesenchymal stem cells. J Mol Cell Cardiol. 2008;44(6):1072–1084. doi: 10.1016/j.yjmcc.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 25.Mayfield AE, Tilokee EL, Latham N, McNeill B, Lam B-K, Ruel M, Suuronen EJ, Courtman DW, Stewart DJ, Davis DR. The effect of encapsulation of cardiac stem cells within matrix-enriched hydrogel capsules on cell survival, post-ischemic cell retention and cardiac function. Biomaterials. 2014;35(1):133–142. doi: 10.1016/j.biomaterials.2013.09.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalcioglu ZI, Mrozek RA, Mahmoodian R, VanLandingham MR, Lenhart JL, Van Vliet KJ. Tunable mechanical behavior of synthetic organogels as biofidelic tissue simulants. J Biomech. 2013;46(9):1583–1591. doi: 10.1016/j.jbiomech.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 27.Engler AJ, Griffin MA, Sen S, Bönnemann CG, Sweeney HL, Discher DE. Myotubes differentiate optimally on substrates with tissue-like stiffness. J Cell Biol. 2004;166(6):877. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kshitiz, Hubbi ME, Ahn EH, Downey J, Afzal J, Kim D-H, Rey S, Chang C, Kundu A, Semenza GL. Matrix Rigidity Controls Endothelial Differentiation and Morphogenesis of Cardiac Precursors. Sci Signaling. 2012;5(227):ra41. doi: 10.1126/scisignal.2003002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Engler AJ, Carag-Krieger C, Johnson CP, Raab M, Tang H-Y, Speicher DW, Sanger JW, Sanger JM, Discher DE. Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating. J Cell Sci. 2008;121(22):3794–3802. doi: 10.1242/jcs.029678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chatterjee K, Lin-Gibson S, Wallace WE, Parekh SH, Lee YJ, Cicerone MT, Young MF, Simon CG., Jr The effect of 3D hydrogel scaffold modulus on osteoblast differentiation and mineralization revealed by combinatorial screening. Biomaterials. 2010;31(19):5051–5062. doi: 10.1016/j.biomaterials.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams C, Budina E, Stoppel WL, Sullivan KE, Emani S, Emani SM, Black LD., III Cardiac extracellular matrix-fibrin hybrid scaffolds with tunable properties for cardiovascular tissue engineering. Acta Biomater. 2015;14:84–95. doi: 10.1016/j.actbio.2014.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kan L, Thayer P, Fan H, Ledford B, Chen M, Goldstein A, Cao G, He J-Q. Polymer microfiber meshes facilitate cardiac differentiation of c-kit+ human cardiac stem cells. Exp Cell Res. 2016;347(1):143–152. doi: 10.1016/j.yexcr.2016.07.024. [DOI] [PubMed] [Google Scholar]
- 33.Zhang J, Huang C, Wu P, Yang J, Song T, Chen Y, Fan X, Wang T. Differentiation induction of cardiac c-kit positive cells from rat heart into sinus node-like cells by 5-azacytidine. Tissue Cell. 2011;43(2):67–74. doi: 10.1016/j.tice.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 34.Boopathy AV, Che PL, Somasuntharam I, Fiore VF, Cabigas EB, Ban K, Brown ME, Narui Y, Barker TH, Yoon Y-S, et al. The modulation of cardiac progenitor cell function by hydrogel-dependent Notch1 activation. Biomaterials. 2014;35(28):8103–8112. doi: 10.1016/j.biomaterials.2014.05.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kurazumi H, Kubo M, Ohshima M, Yamamoto Y, Takemoto Y, Suzuki R, Ikenaga S, Mikamo A, Udo K, Hamano K, et al. The Effects of Mechanical Stress on the Growth, Differentiation, and Paracrine Factor Production of Cardiac Stem Cells. PLoS One. 2011;6(12):e28890. doi: 10.1371/journal.pone.0028890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hausenloy DJ, Yellon DM. Cardioprotective growth factors. Cardiovasc Res. 2009;83(2):179. doi: 10.1093/cvr/cvp062. [DOI] [PubMed] [Google Scholar]
- 37.Cross MJ, Claesson-Welsh L, et al. FGF and VEGF function in angiogenesis: signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol Sci. 2001;22(4):201–207. doi: 10.1016/s0165-6147(00)01676-x. [DOI] [PubMed] [Google Scholar]
- 38.Zhang G-W, Gu T-X, Guan X-Y, Sun X-J, Jiang D-Q, Tang R, Qi X, Li X-Y. Delayed Enrichment for c-kit and Inducing Cardiac Differentiation Attenuated Protective Effects of BMSCs’ Transplantation in Pig Model of Acute Myocardial Ischemia. Cardiovasc Ther. 2015;33(4):184–192. doi: 10.1111/1755-5922.12131. [DOI] [PubMed] [Google Scholar]
- 39.Zhang LX, DeNicola M, Qin X, Du J, Ma J, Tina Zhao Y, Zhuang S, Liu PY, Wei L, Qin G, et al. Specific inhibition of HDAC4 in cardiac progenitor cells enhances myocardial repairs. Am J Physiol Cell Physiol. 2014;307(4):C358–72. doi: 10.1152/ajpcell.00187.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Funakoshi S, Miki K, Takaki T, Okubo C, Hatani T, Chonabayashi K, Nishikawa M, Takei I, Oishi A, Narita M, et al. Enhanced engraftment, proliferation, and therapeutic potential in heart using optimized human iPSC-derived cardiomyocytes. Sci Rep. 2016;6:19111. doi: 10.1038/srep19111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oshima H, Payne TR, Urish KL, Sakai T, Ling Y, Gharaibeh B, Tobita K, Keller BB, Cummins JH, Huard J. Differential myocardial infarct repair with muscle stem cells compared to myoblasts. Mol Ther. 2005;12(6):1130–1141. doi: 10.1016/j.ymthe.2005.07.686. [DOI] [PubMed] [Google Scholar]
- 42.Ernst C, Christie BR. Isolectin-IB4 as a vascular stain for the study of adult neurogenesis. J Neurosci Methods. 2006;150(1):138–142. doi: 10.1016/j.jneumeth.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 43.Quevedo HC, Hatzistergos KE, Oskouei BN, Feigenbaum GS, Rodriguez JE, Valdes D, Pattany PM, Zambrano JP, Hu Q, McNiece I, et al. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc Natl Acad Sci U S A. 2009;106(33):14022–14027. doi: 10.1073/pnas.0903201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu Y, Ip JE, Huang J, Zhang L, Matsushita K, Liew C-C, Pratt RE, Dzau VJ. Essential role of ICAM-1/CD18 in mediating EPC recruitment, angiogenesis, and repair to the infarcted myocardium. Circ Res. 2006;99(3):315–322. doi: 10.1161/01.RES.0000235986.35957.a3. [DOI] [PubMed] [Google Scholar]
- 45.Cheng K, Li T-S, Malliaras K, Davis DR, Zhang Y, Marbán E. Magnetic Targeting Enhances Engraftment and Functional Benefit of Iron-Labeled Cardiosphere-Derived Cells in Myocardial Infarction. Circ Res. 2010;106(10):1570. doi: 10.1161/CIRCRESAHA.109.212589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Terrovitis JV, Smith RR, Marbán E. Assessment and optimization of cell engraftment after transplantation into the heart. Circ Res. 2010;106(3):479–494. doi: 10.1161/CIRCRESAHA.109.208991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee TT, García JR, Paez JI, Singh A, Phelps EA, Weis S, Shafiq Z, Shekaran A, Del Campo A, García AJ. Light-triggered in vivo activation of adhesive peptides regulates cell adhesion, inflammation and vascularization of biomaterials. Nat Mater. 2015;14(3):352–360. doi: 10.1038/nmat4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ip JE, Wu Y, Huang J, Zhang L, Pratt RE, Dzau VJ. Mesenchymal stem cells use integrin beta1 not CXC chemokine receptor 4 for myocardial migration and engraftment. Mol Biol Cell. 2007;18(8):2873–2882. doi: 10.1091/mbc.E07-02-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.