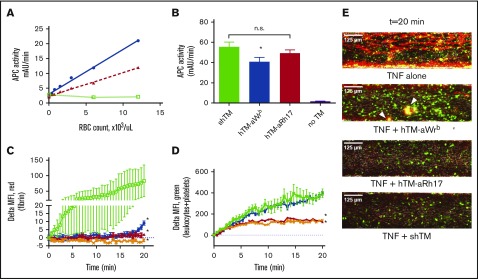
Figure 6.
Characterization of the activity of RBCs bound by hTM-scFv fusions and their therapeutic efficacy in a microfluidic model of inflammatory thrombosis. (A) APC generation by RBCs loaded with hTM-scFv demonstrates a dose- and copy-number–dependent response in APC generation as measured by chromogenic assay. hTM-aBand3 (blue circles) showed about twofold higher APC generation per RBC compared with hTM-aRhCE (red triangles), although copy numbers are expected to be fivefold to 10-fold higher. Soluble hTM (shTM)-treated RBCs are shown as a nonbinding control (green open squares). (B) Comparison of APC-generative capacity of sTM vs hTM-scFv fusions (added at 50 nM) in a high Hct (20%) RBC suspension. Mean ± SD is shown; n = 3 for each condition (*P < .05 vs sTM, 1-way ANOVA with Holm-Sidak correction for multiple comparisons). A slight reduction in activity was seen for hTM-aBand3 but not hTM-aRhCE. (C) Fibrin generation on TNF-α–activated, endothelialized microfluidic channels perfused with human whole blood preincubated with either PBS control (green open squares), shTM control (orange crosses), hTM-Wrb (blue circles), or hTM-aRh17 (red triangles). Both fusion proteins (and shTM+ control) significantly reduced fibrin generation (*P < .05 vs untreated, 1-way ANOVA with Holm-Sidak correction for multiple comparisons) as compared with the control channel. An increase in fibrin generation was noted toward the end of the observation period for the hTM-aWrb–treated channels. (D) hTM-aRh17 treatment (red triangles) more effectively reduced platelet and leukocyte adhesion (quantified with calcein AM fluorescence) than hTM-aWrb (blue circles) vs untreated control (open squares). hTM-Rh17 treatment was similar to shTM+ control (crosses). For panels C-D, mean ± standard error of the mean (SEM) for 2 independent channels is shown. (E) Representative composite images of whole blood (fibrin in red [anti-fibrin-AlexaFluor568 stain], platelets and leukocytes in green [calcein AM stain], brightfield image in gray) flowing through endothelialized channels at the end of the observation period (t = 20 minutes). Fibrin is decreased in both fusion-treated channels. An increase in platelet adhesion with associated fibrin (yellow, arrowhead) is seen in the hTM-aWrb–treated channels compared with hTM-aRh17 (see supplemental Videos 1-4 for the full time course).