Fig. 2. Regulation of Runx2 gene promoter activity and mRNA levels after mitosis.
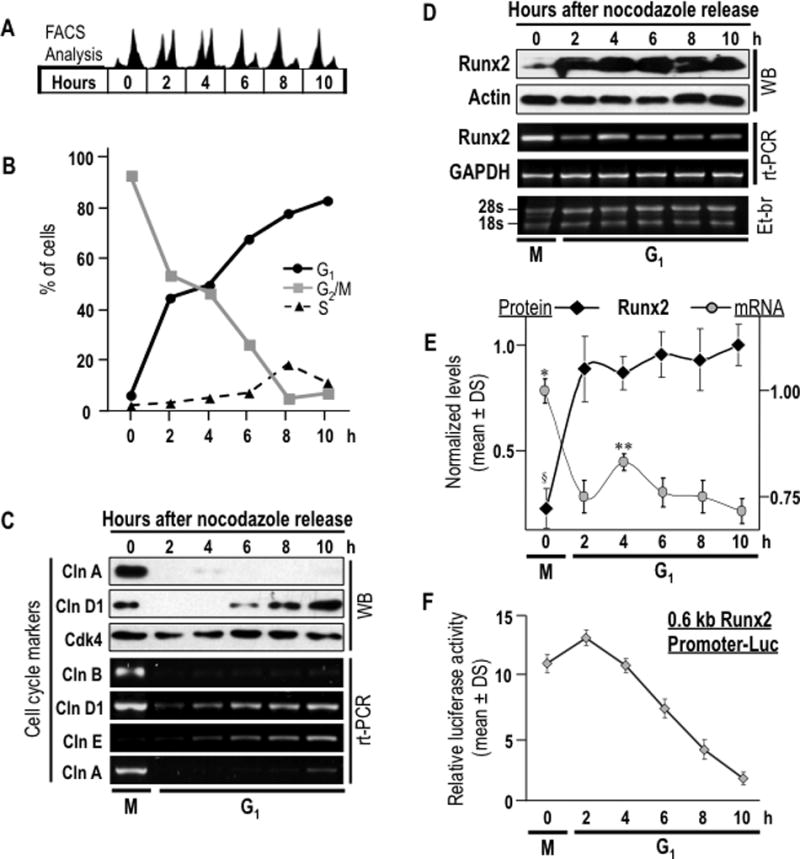
Runx2 protein and mRNA levels as well as Runx2 promoter activity were assessed during M/G1 phase transition in pre-osteoblasts MC3T3-E1 cells to analyze post-mitotic modulation of Runx2 mRNA levels. (A) Cells were synchronized by incubation for 16 h with nocodazole to generate a mitotic block. Mitotic cells were collected by gentle agitation (‘mitotic shake-off’), replated and released from mitosis into G1 by addition of fresh culture medium. Cells were harvested after 0, 2, 4, 6, and 10 h. Progression through mitosis into the next G1 phase was monitored by flow cytometry. (B) Graphic representation of cell cycle stage data presented in panel A. (C) Expression of cell cycle markers was evaluated by western blot analysis (cyclins A, D and Cdk4) and RT-PCR (cyclins D, E, A and B). (D) Post-mitotic modulations in Runx2 protein and mRNA levels were assessed by western blot and RT-PCR analyses. (E) Graphic representation of post-mitotic changes in Runx2 protein (§P = 0,003 between time 0 h, mitosis, and times 2 to 10 h, G1 phase) and mRNA levels (*P = 0.001 between 0 h, mitosis, and times 2 to 10 h, G1 phase; **P = 0.030 between 4 h and 2 h, and P = 0.03 between 4 h and 6 h). Datapoints represent the averages and standard deviation of multiple experiments. Protein and mRNA values were normalized to actin and GAPDH, respectively. (F) Relative promoter activity of Runx2 promoter/luciferase reporter gene construct (mouse 0.6 kB/LUC) is plotted. Luciferase values were normalized to SV40/Ranilla construct activity. Values are the means of three distinct experiments. Onset of the M/G1 phase transition as determined by flow cytometry is indicated at the bottom of the gels and graphs.
