Fig. 6. Restitution of Runx2 protein levels during early G1 phases of cell cycle is independent of transcriptional activity for mRNA synthesis.
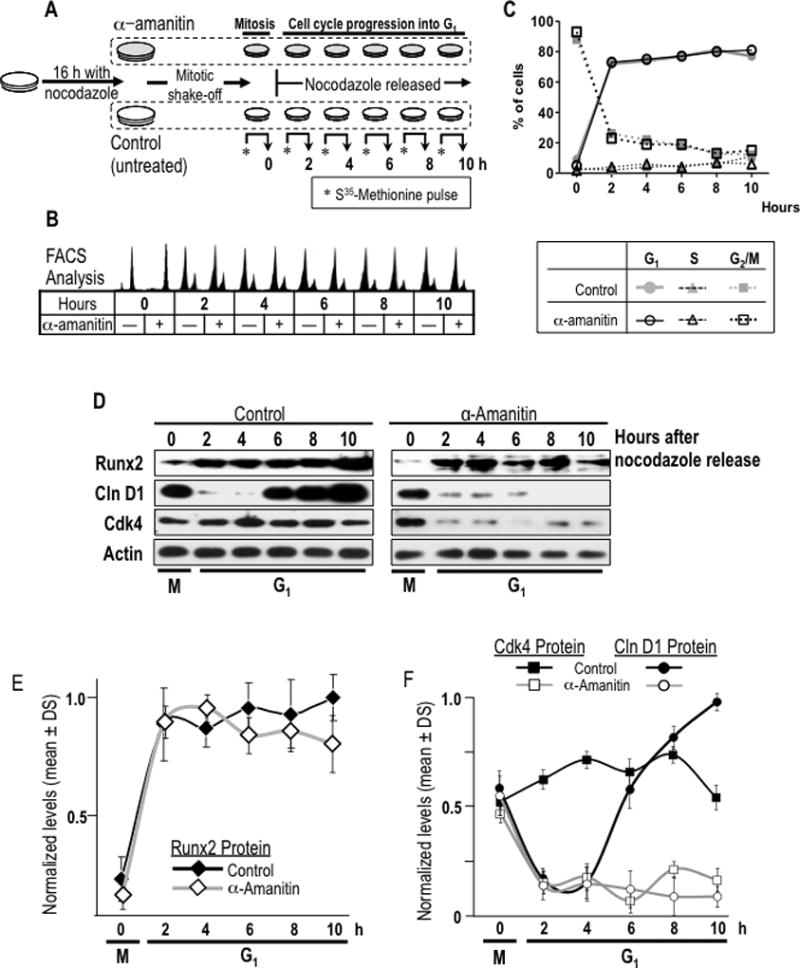
(A) A specific experimental strategy using the RNA polymerase II inhibitor α-amanitin was developed to block the post-mitotic contribution of mRNA synthesis to Runx2 protein synthesis during G1. MC3T3-E1 cells were synchronized by incubation for 16 h with nocodazole to generate a mitotic block. Mitotic cells were pre-treated with or without α-amanitin for 2 h and harvested by gentle agitation (0 h) and then released through mitosis into G1 by washing and the addition of fresh culture medium supplemented with (+) or without (−) α-amanitin. (B) Cells were harvested at selected time points during the M/G1 phase transition and interphase (0, 2, 4, 6, and 10 h). Progression through mitosis to G1 phase in presence or absence of α-amanitin was monitored by flow cytometry. (C) Graphic representation of cell cycle stage data presented in panel B shown that α-amanitin treatment does not affect normal cell cycle progression of mitotic cells into G1 phase. (D) Resumption of Runx2 protein levels at the M/G1 transition in α-amanitin-treated cells or untreated control cells was analysed by western blotting. Cell cycle progression into G1 was defined by monitoring cyclin D1 and Cdk4 protein levels. (E and F) Graphic representations of α-amanitin-treatment affecting post-mitotic change in Runx2 (no statistical differences between control and treated group), cyclin D1 (significant statistical differences between control and treated group from 6 h, P = 0.001) and Cdk4 (significant statistical differences between control and treated group from 2 h, P = 0.001). Protein levels normalized to actin.
