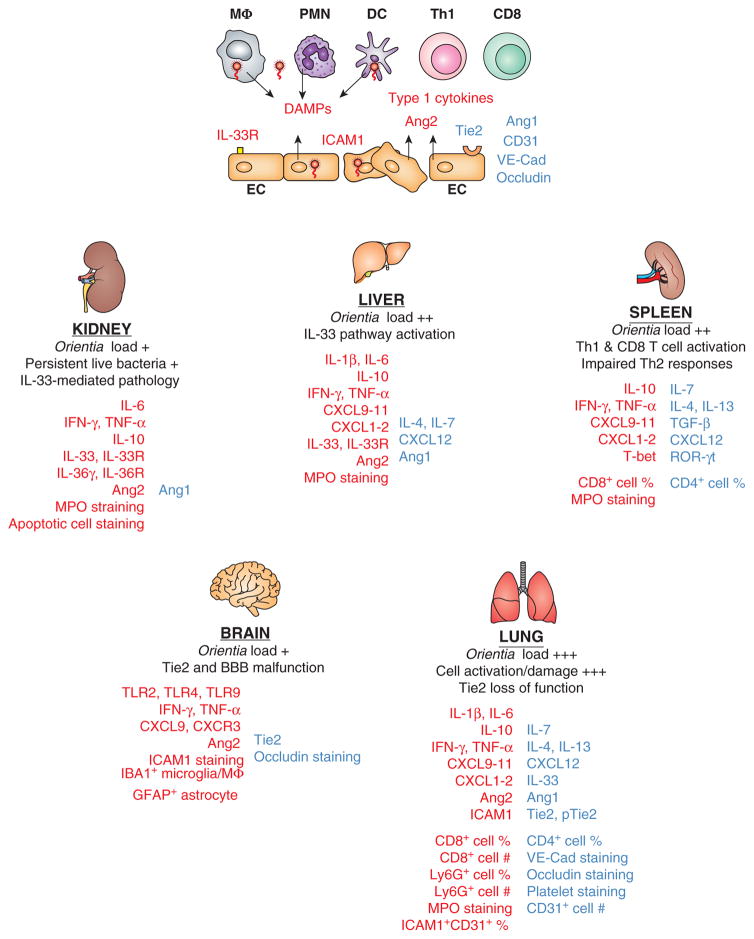
Figure 1. Dysregulated Th1 immune responses, accompanied by impaired Th2 responses, contribute to Orientia tsutsugamushi-induced acute tissue injury and vascular dysfunction.
Biomarkers or cell subsets that are up-regulated (in red) or down-regulated (in blue) during in vitro infection of human cell cultures and lethal or sublethal infection in C57BL/6 mice are illustrated. At the cellular level (top panel of the figure), Orientia coccobacilli within target cells such as monocytes/macrophages (MΦ), dendritic cells (DC), neutrophils (PMN), and endothelial cells (EC) preferentially stimulate the activation of Th1 and CD8+ T cells, but not Th2 cells, with high levels of type 1/inflammatory cytokines. Infected target cells can also release or activate damage-associated molecular pattern (DAMP) molecules such as IL-33 and other host factors. ECs can contribute to immune activation via surface expression of IL-33 receptors and ICAM1, or the release of vascular destabilizing factors such as angiopoietin protein-2 (Ang2). Sustained infection can trigger EC apoptosis and down-regulate the expression of Ang1, CD31 (also known as platelet endothelial cell adhesion molecule-1), occludin (a tight junction-associated protein), and VE-cadherin (endothelial adhesion junctions) via yet-undefined mechanisms. At the tissue level (lower panels of the figure), bacterial loads at the acute or chronic stages of disease are illustrated comparatively, together with hallmarks of pathological changes in the examined organs. The common immunological changes revealed from recent publications (65, 71, 75–78) include the sustained production of Th1 cytokines, selective activation of CD8+ T cells and Ly6G/MPO-positive PMNs, as well as organ-specific differences in the IL-33-like DAMPs. Vascular dysfunction is presented as reduced expression of Ang1, Tie2 (a tyrosine-protein kinase receptor for Ang1 and Ang2), CD31, occludin, and VE-cadherin at the severe stages of infection in mice. Data for lung flow cytometry and vascular staining, as well as kidney IL-36γ/IL-36R analysis, are unpublished. Collectively, these cell- and tissue-specific alterations contribute to the loss of vascular integrity, excessive tissue damage, and host mortality.