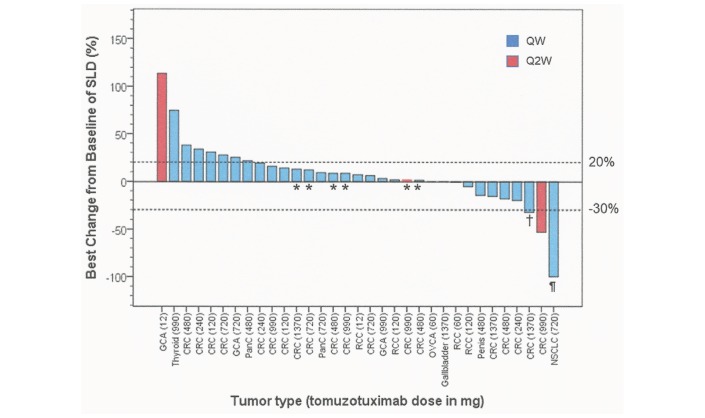
Figure 2.
Waterfall plot of the best per cent change from baseline in SLD of target lesions in 33 patients. Baseline is defined as the last non-missing value before the first dose of tomuzotuximab. Only patients with valid baseline and postbaseline values are included. Tumour assessment was not performed in six patients because of early withdrawal from the study following clinical deterioration or AE; two patients had no measurable disease according to RECIST1.1 criteria. The dotted lines indicate the cut-off for partial response (−30%) and progressive disease (+20%). *Patients with stable target lesions but progression because of new lesions. †Patient (CRC, dose 1370 mg) had a best change from baseline of 32.2%, unconfirmed 50 days later (change from baseline 29.5%). ¶ Patient is still in complete remission (4.5 years) and received tomuzotuximab for 5.2 years. CRC, colorectal cancer; GCA, gastric cancer; NSCLC, non-small cell lung cancer; OVCA, ovarian cancer; PanC, pancreatic cancer; RCC, renal cell cancer; SLD, sum of longest diameters.