Abstract
Background
Respiratory rate (RR) is an independent predictor of adverse outcomes and an integral component of many risk prediction scores for hospitalised adults. Yet, it is unclear if RR is recorded accurately. We sought to assess the potential accuracy of RR by analysing the distribution and variation as a proxy, since RR should be normally distributed if recorded accurately.
Methods
We conducted a descriptive observational study of electronic health record data from consecutive hospitalisations from 2009 to 2010 from six diverse hospitals. We assessed the distribution of the maximum RR on admission, using heart rate (HR) as a comparison since this is objectively measured. We assessed RR patterns among selected subgroups expected to have greater physiological variation using the coefficient of variation (CV=SD/mean).
Results
Among 36 966 hospitalisations, recorded RR was not normally distributed (p<0.001), but right skewed (skewness=3.99) with values clustered at 18 and 20 (kurtosis=23.9). In contrast, HR was relatively normally distributed. Patients with a cardiopulmonary diagnosis or hypoxia only had modestly greater variation (CV increase of 2%–6%). Among 1318 patients transferred from the ward to the intensive care unit (n=1318), RR variation the day preceding transfer was similar to that observed on admission (CV 0.24 vs 0.26), even for those transferred with respiratory failure (CV 0.25).
Conclusions
The observed patterns suggest that RR is inaccurately recorded, even among those with cardiopulmonary compromise, and represents a ‘spot’ estimate with values of 18 and 20 breaths per minute representing ‘normal.’ While spot estimates may potentially be adequate to indicate clinical stability, inaccurate RR may alternatively lead to misclassification of disease severity, potentially jeopardising patient safety. Thus, we recommend greater training for hospital personnel to accurately record RR.
INTRODUCTION
Respiratory rate (RR) is an independent predictor of mortality, intensive care unit (ICU) admission and cardiac arrest across a variety of conditions among hospitalised adults.1–8 It is also an integral component of many risk prediction scores such as the modified early warning system (MEWS)8,9 and is one of the clinical criteria for determining the stability for discharge.10,11 While certain risk prediction scores use the RR as a dichotomous risk factor in the tachypnoea range (ie, RR ≥22 for the quick Sequential [Sepsis-related] Organ Failure Assessment (qSOFA)),4–6 others have shown prognostic value across the entire spectrum of RR values.3,7,8 Thus, inaccurately recorded RR may lead to misclassification of disease severity and bias commonly used risk prediction scores used both by clinicians as well as electronic health record (EHR)-based surveillance systems.12,13
While other vital signs such as heart rate (HR) are measured objectively using automated technology, RR is visually assessed and potentially subject to greater imprecision and error. The criterion ‘gold’ standard for measurement of RR is to visually observe or auscultate the chest to count breaths for 1 min, or at a minimum, for 30 s with multiplication of the number of observed breaths by 2 to obtain breaths per minute.14–17 Compared with the true observed RR using the criterion standard technique, which follows a relatively normal distribution, RRs recorded in usual care settings have been shown to be inaccurate.14,18–20 However, these studies were mostly small, single-centre cohorts conducted in an emergency department setting.14,18 To our knowledge, the only study conducted among hospitalised adults was modestly sized (only 368 patients), occurred within a single day and included only academic medical centres, limiting the robustness and generalisability of these findings.19
Thus, we sought to assess the potential accuracy of the recorded RR in a large, diverse, multicentre cohort of hospitalised adults by analysing the distribution and patterns of variation in RR as a proxy for accuracy, since recorded RR would be expected to be normally distributed like other physiological parameters (ie, HR) if recorded accurately, based on the known distribution of RR from prior research.19,20 We hypothesised that recorded RR would be non-normally distributed with clustering of values between 16 and 20, suggesting inaccurate recording of values. However, among key subgroups where the accuracy of RR is more important for clinical decision making and higher values would be expected (eg, cardiopulmonary diagnoses), we hypothesised there would be greater variation in recorded RR.
METHODS
We conducted a retrospective observational cohort study of EHR data from consecutive medical hospitalisations among adults ≥18 years old from November 2009 to October 2010 from six diverse hospitals (academic, community and safety-net hospitals). Details of this cohort have been published previously.21
We excluded patients admitted directly to the ICU since RR is measured differently than on the medicine floor (eg, patients may be mechanically ventilated and the RR predetermined by the ventilator settings). EHR data on RR and HR were recorded as part of usual care. For each hospital day, we extracted the minimum and maximum values. To mitigate the effect of extreme outlier values and potential errors, we Winsorised the RR, setting all values above the 99th percentile equal to the value at that percentile (60 breaths per minute). As a proxy for accuracy, we assessed the distribution of the RR using histograms, skewness and kurtosis using HR as a comparison since it is an objectively measured vital sign. A normal distribution would have a skewness of 0 and kurtosis of 3, with larger values indicating non-normal distribution. We assessed variation in the recorded RR using the coefficient of variation (CV=SD/mean). Lastly, we assessed patterns and variation of RR among selected subgroups expected to have higher values and more physiological variation (ie, day of hospitalisation, cardiopulmonary diseases, hypoxia, day prior to ICU transfer, age and sex) or potential differences in resource availability (hospital type: public vs non-public). We qualitatively evaluated the histogram for normality and differences in the CV because our large sample size would lead to deviations or differences considered statistically significant even if not clinically meaningful.
RESULTS
We included 36 966 hospitalisations among 28 511 patients, representing 220 665 unique hospital days (see online supplementary figure for study flow diagram). The mean age was 62 years, 54% were female, 40% were non-white, and the median length of stay was 4 days (table 1).
Table 1.
Characteristics of included hospitalisations
| (n=36 966) | |
|---|---|
| Age in years (mean +/− SD) | 61.7 (17.4) |
| ≥65 years old (%) | 17 237 (46.9) |
| Female, n (%) | 20 064 (54.3) |
| Ethnicity, n (%) | |
| White | 23 042 (62.3) |
| Black | 7241 (19.6) |
| Hispanic | 5499 (14.9) |
| Other | 1184 (3.2) |
| Hospital type, n (%) | |
| County | 9328 (25.2) |
| Non-county | 27 638 (74.8) |
| Length of stay, median (IQR) | 4 (2–6) |
| Charlson comorbidity index*, n (%) | |
| 0 | 22 233 (60.1) |
| 1 | 2682 (7.3) |
| 2+ | 12 051 (32.6) |
| Cardiopulmonary primary discharge diagnosis†, n (%) | 5814 (15.7) |
| Sepsis | 656 (1.8) |
| Pneumonia | 1431 (3.9) |
| COPD exacerbation | 787 (2.1) |
| CHF exacerbation | 2003 (5.4) |
| Asthma exacerbation | 423 (1.1) |
| Myocardial infarction | 514 (1.4) |
| Oxygen saturation, minimum value on admission, n (%) | |
| ≥92% | 30 481 (82.5) |
| <92% | 5860 (15.8) |
| Missing oxygen saturation | 625 (1.7) |
Charlson comorbidity index was calculated using the Deyo modification.
Classified using the AHRQ Clinical Classifications Software.
CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease.
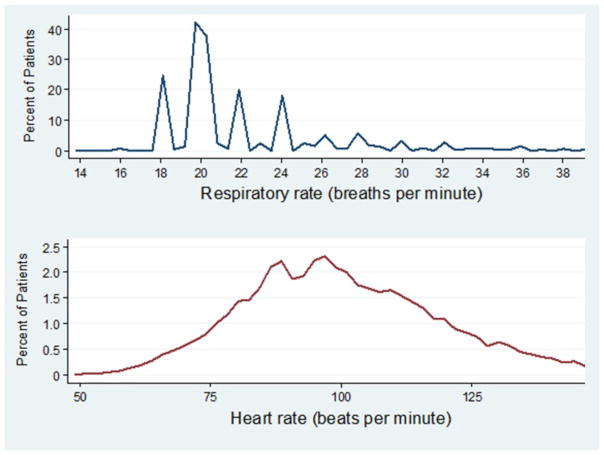
The maximum recorded RR values upon admission were not normally distributed (p<0.001 for the joint skewness and kurtosis test of normality), but were right skewed (skewness=3.99), with values clustered at 18 and 20 breaths per minute (kurtosis=23.92). In contrast, HR was relatively normally distributed (figure 1). The minimum RR equalled the maximum value in 26% of hospital days. The maximum RR equalled 18 or 20 in 75% of all hospital days. RR variation decreased substantially over the duration of hospitalisation (CV 0.26 on the first day, 0.21 2 days prior to discharge, 0.16 on day of discharge; table 2). However, patients with cardiopulmonary illness (CV 0.27 vs 0.25) or hypoxia (CV 0.30 vs 0.24) had only modestly greater variation in RRs than those without. The increase in variability in these two subgroups was due to more individuals with elevated RR, given higher median and 75th percentile values for RR, with identical 25th percentile values of 20 compared with patients without a cardiopulmonary diagnosis or hypoxia. Among all patients transferred from the general medicine ward to the ICU (n=1318), distribution and variation of the maximum RR the day prior to transfer was similar to that observed on admission, even for those transferred to the ICU with respiratory failure. Lastly, average RR values and variation between age, sex and hospital-type groups were similar.
Figure 1.
Distribution of maximum recorded respiratory rate and heart rate on day of admission among hospitalised adults.
Table 2.
Variation of recorded RRs (breaths per minute) among selected subgroups
| Results by key subgroups | Mean (SD) | Median (IQR) | CV (SD/mean) |
|---|---|---|---|
| Hospital day | |||
| Day of admission | |||
| Maximal RR | 22.0 (5.7) | 20 (20–22) | 0.26 |
| Minimal RR | 15.9 (2.3) | 16 (16–18) | 0.14 |
| 2 days before discharge (maximal RR)* | 20.3 (4.2) | 20 (18–20) | 0.21 |
| Day of discharge (maximal RR) | 19.3 (3.1) | 20 (18–20) | 0.16 |
| Principal diagnosis† | |||
| Cardiopulmonary diagnosis‡ | 24.4 (6.5) | 22 (20–26) | 0.27 |
| Non-cardiopulmonary diagnosis | 21.6 (5.4) | 20 (20–22) | 0.25 |
| Oxygen saturation† | |||
| <92% | 24.6 (7.3) | 22 (20–26) | 0.30 |
| ≥92% | 21.6 (5.2) | 20 (20–22) | 0.24 |
| Prior to ICU transfer§ | |||
| Patients with any diagnosis | 21.2 (5.1) | 20 (20–22) | 0.24 |
| Patients with respiratory failure | 22.3 (5.5) | 20 (20–24) | 0.25 |
| Age† (years) | |||
| ≥65 | 22.3 (5.9) | 20 (20–24) | 0.26 |
| <65 | 21.8 (5.5) | 20 (20–22) | 0.25 |
| Sex† | |||
| Female | 22.0 (5.6) | 20 (20–22) | 0.25 |
| Male | 21.1 (5.8) | 20 (20–23) | 0.26 |
| Hospital type† | |||
| Public hospital | 21.9 (5.5) | 20 (20–22) | 0.25 |
| Non-public hospital | 22.1 (5.8) | 20 (20–22) | 0.26 |
Restricted to patients with hospital length of stay ≥4 days (n=26 223 hospitalisations).
Maximum recorded RR on the first day of admission.
Cardiopulmonary diseases include pneumonia, sepsis, COPD exacerbation, asthma exacerbation, myocardial infarction and CHF exacerbation based on the AHRQ Clinical Classification Software.
Maximum recorded RR for all patients on the day prior to transfer to the ICU from the general medicine wards (n=1318), and for the 352 patients the day prior to ICU transfer with a primary or secondary discharge diagnosis for respiratory failure.
CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CV, coefficient of variation; ICU, intensive care unit; RR, respiratory rate.
DISCUSSION
In a large, diverse, multicentre cohort of adults hospitalised for a broad range of medical conditions, we found that the recorded RR was not normally distributed, and that there was little variation in the recorded RR, even among those with cardiopulmonary compromise or immediately prior to ICU transfer. The clustering of values and right-skewed pattern suggests that the recorded RR represents an estimated or ‘spot’ measurement, with values of 18 and 20 breaths per minute representing ‘normal.’
Our overall findings are consistent with other smaller prospective studies which found that the recorded RR was inaccurate compared with the criterion standard technique for measuring RR.14,18–20 In addition to including a larger and more generalisable cohort, our study extends on this work by showing that the distribution of RR among key subgroups expected to have more variation in RR due to physiological differences was nonetheless fairly similar (ie, age).20 Even among those with hypoxia or a cardiopulmonary diagnosis, conditions expected to markedly increase the RR and correspondingly confer greater risk for respiratory failure and clinical deterioration, we only observed modest increases (2%–6%) in the variation of the recorded RR. Similarly, the very modest degree of variation in recorded RR the day prior to ICU transfer is concerning because of the possibility that early signs of respiratory failure may have been missed due to inaccurate measurement technique. Lastly, we found that the RR distribution was virtually identical for patients irrespective of hospital setting, suggesting that the practice of estimating rather than directly observing the RR may not be simply due to limited resources such as suboptimal nursing assistant to patient ratios.
Despite the limited overall variability, our findings suggest that there is greater attention to the RR when it is abnormally high, given the right-skewed distribution, greater variation on the day of admission (when patients are expected to have greater acuity and severity of illness) and greater variation in the maximum recorded value compared with the minimum value. While RR has a prognostic value when abnormally elevated,4,5,10,11,21 it is less clear if the same holds true for RR within the ‘normal’ range. Thus, ‘spot’ assessments for RR using a value of 18 or 20 for ‘normal’ may be sufficient to indicate clinical stability.
Alternatively, inaccurate RRs may lead to misclassification of disease severity, stability and prognosis. For example, Escobar and colleagues found that every 1 breath increase in the recorded RR among hospitalised adults on the wards was predictive of ICU transfer or death outside of the ICU, even within the normal range.7 Given that this study used RR values from the EHR, which are likely prone to inaccuracies similar to those in our cohort, the true prognostic value of RR for predicting clinical decompensation may be even greater if RR was recorded accurately using the criterion standard technique. Thus, clinicians and EHR-based surveillance systems could be alerted sooner to potentially concerning trends in the RR (eg, RR increasing from 12 to 14 to 20) in advance without waiting for the patient to become overtly tachypnoeic (RR >20). These may represent missed opportunities for intervention with potentially detrimental effects on patient safety especially for patients with cardiopulmonary compromise.
Another potential consequence of using ‘spot’ assessments is that EHR-based surveillance systems may trigger false alarms of impending deterioration among truly stable hospitalised patients. For example, using 18 or 20 breaths per minute to represent ‘normal’ will artificially inflate certain risk prediction scores such as the MEWS (0 point for 9–14 breaths per minute vs 1 point for 15–20 breaths per minute on a 13-point scale). Thus, patients may be falsely identified as being ‘unstable’ and trigger rapid response team alerts, leading to alarm fatigue as well as misallocation of valuable hospital personnel and resources evaluating these stable patients.
The main limitation of this study was the lack of an objective measure of RR, limiting our ascertainment of whether individual RRs were correctly measured. However, the lack of normal distribution, predominance of values clustered at 18 and 20, and only minimal differences in variation by clinical condition and hypoxemia are ample surrogates for the validity of our findings.
Future research should examine whether measuring RR accurately, particularly in the normal range, improves prognostication, triage decisions and outcomes among hospitalised adults. Nonetheless, we suggest greater education and training for clinical hospital personnel to use the criterion standard technique for recording the RR. The additional 30–60 s per RR assessment could be performed concurrently while obtaining automated assessments for the remaining vital signs without requiring any additional resources or time. Future research and quality improvement efforts should confirm whether this can be accomplished within the current workflow without overburdening the staff. Finally, for decisions that hinge on a high-fidelity assessment of respiratory status (such as sepsis and pneumonia), we encourage clinicians to count the RR rather than simply relying on the recorded RR in the EHR.
Supplementary Material
Acknowledgments
Funding This work was supported by the Agency for Healthcare Research and Quality-funded UT Southwestern Center for Patient-Centered Outcomes Research (R24 HS022418-01); the Commonwealth Foundation (#20100323); the UT SouthwesternKL2 Scholars Program supported by the National Institutes of Health (KL2TR001103); the National Center for Advancing Translational Sciences at the National Institute of Health (U54 RFA-TR-12-006); and the National Institute on Aging (K23AG052603). The study sponsors had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The authors have no conflicts of interest to disclose, financial or otherwise.
Footnotes
Contributors Conception or design of the work: All authors. Data collection: CC, EAH. Data analysis and interpretation: CC, AM, JB. Drafting the article: JB, AM. Critical revision of the article: all authors. Final approval of the version to be published: all authors.
Competing interests None declared.
Patient consent The IRBs exempted the consent requirement since it was a retrospective study on electronic health records and did not contain identifiable information.
Ethics approval The IRBs of both UT Southwestern and Texas Health Resources.
Provenance and peer review Not commissioned; externally peer reviewed.
Additional material is published online only. To view please visit the journal online (http://dx.doi.org/10.1136/bmjqs-2017-006671).
References
- 1.Churpek MM, Yuen TC, Huber MT, et al. Predicting cardiac arrest on the wards: a nested case-control study. Chest. 2012;141:1170–6. doi: 10.1378/chest.11-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fieselmann JF, Hendryx MS, Helms CM, et al. Respiratory rate predicts cardiopulmonary arrest for internal medicine inpatients. J Gen Intern Med. 1993;8:354–60. doi: 10.1007/BF02600071. [DOI] [PubMed] [Google Scholar]
- 3.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–29. [PubMed] [Google Scholar]
- 4.Bone RC, Balk RA, Cerra FB, et al. Definitions for Sepsis and Organ failure and guidelines for the use of innovative therapies in Sepsis. Chest. 1992;101:1644–55. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 5.Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the third International consensus sefinitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:762–74. doi: 10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336:243–50. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 7.Escobar GJ, LaGuardia JC, Turk BJ, et al. Early detection of impending physiologic deterioration among patients who are not in intensive care: development of predictive models using data from an automated electronic medical record. J Hosp Med. 2012;7:388–95. doi: 10.1002/jhm.1929. [DOI] [PubMed] [Google Scholar]
- 8.Morgan R, Williams F, Wright M. An early warning scoring system for detecting developing critical illness. Clin Intensive Care. 1997;8:100. [Google Scholar]
- 9.Ludikhuize J, Brunsveld-Reinders AH, Dijkgraaf MG, et al. Outcomes associated with the nationwide introduction of rapid response systems in the Netherlands. Crit Care Med. 2015;43:2544–51. doi: 10.1097/CCM.0000000000001272. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen OK, Makam AN, Clark C, et al. Vital signs are still vital: instability on Discharge and the risk of Post-Discharge adverse outcomes. J Gen Intern Med. 2017;32:42–8. doi: 10.1007/s11606-016-3826-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halm EA, Fine MJ, Kapoor WN, et al. Instability on hospital discharge and the risk of adverse outcomes in patients with pneumonia. Arch Intern Med. 2002;162:1278–84. doi: 10.1001/archinte.162.11.1278. [DOI] [PubMed] [Google Scholar]
- 12.Bates DW, Zimlichman E. Finding patients before they crash: the next Major opportunity to improve patient safety. BMJ Qual Saf. 2015;24:1–3. doi: 10.1136/bmjqs-2014-003499. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt PE, Meredith P, Prytherch DR, et al. Impact of introducing an electronic physiological surveillance system on hospital mortality. BMJ Qual Saf. 2015;24:176–7. doi: 10.1136/bmjqs-2014-003845. [DOI] [PubMed] [Google Scholar]
- 14.Lovett PB, Buchwald JM, Stürmann K, et al. The vexatious vital: neither clinical measurements by nurses nor an electronic monitor provides accurate measurements of respiratory rate in triage. Ann Emerg Med. 2005;45:68–76. doi: 10.1016/j.annemergmed.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. Fourth Programme Report, 1988–1989: ari Programme for Control of acute respiratory infections. Geneva, Switzerland: WHO; 1990. p. 31. [Google Scholar]
- 16.Bickley LS, Szilagyi PG. Bates’ Guide to physical examination and history taking. 8. Philadelphia: Lippincott Williams & Wilkins; 2003. [Google Scholar]
- 17.Ball J, Jane W. Seidel’s Guide to Physical Examination. St. Louis, Missouri: Elsevier/Mosby; 2014. [Google Scholar]
- 18.Bianchi W, Dugas AF, Hsieh YH, et al. Revitalizing a vital sign: improving detection of tachypnea at primary triage. Ann Emerg Med. 2013;61:37–43. doi: 10.1016/j.annemergmed.2012.05.030. [DOI] [PubMed] [Google Scholar]
- 19.Semler MW, Stover DG, Copland AP, et al. Flash mob research: a single-day, multicenter, resident-directed study of respiratory rate. Chest. 2013;143:1740–4. doi: 10.1378/chest.12-1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodríguez-Molinero A, Narvaiza L, Ruiz J, et al. Normal respiratory rate and peripheral blood oxygen saturation in the elderly population. J Am Geriatr Soc. 2013;61:2238–40. doi: 10.1111/jgs.12580. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen OK, Makam AN, Clark C, et al. Predicting all-cause readmissions using electronic health record data from the entire hospitalization: model development and comparison. J Hosp Med. 2016;11:473–80. doi: 10.1002/jhm.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.