Figure 5. Syncrip binds the long form of HLA-A 3’UTR and inhibits luciferase activity of the constructs.
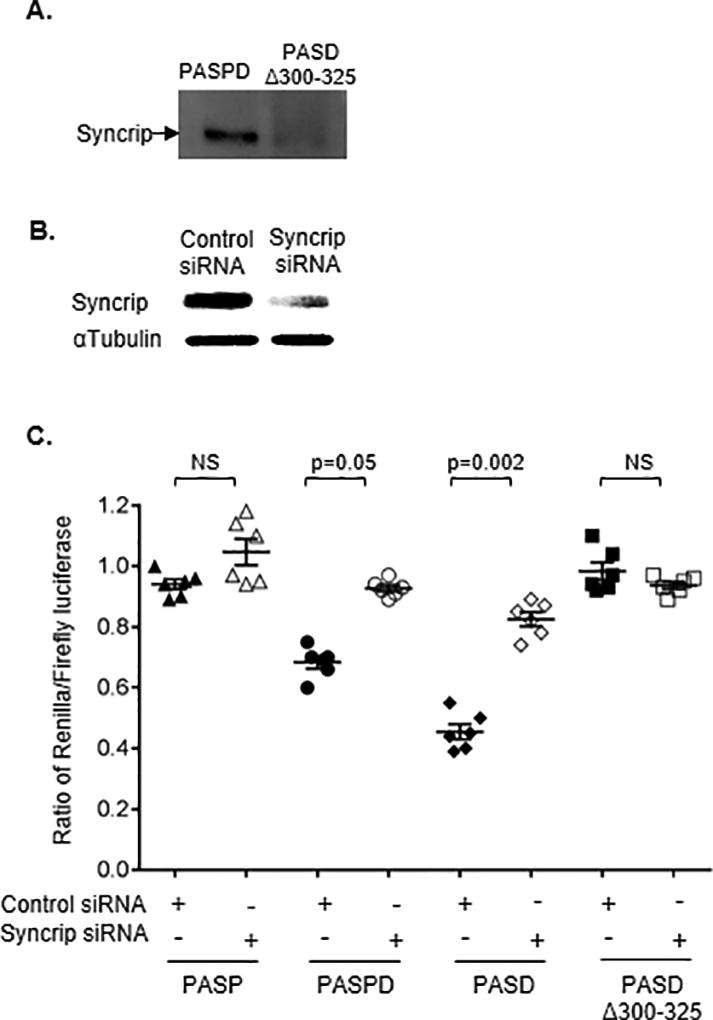
(A) Western blot indicated the presence of Syncrip bound to the long form of HLA-A 3’UTR. RNA affinity pulldown was carried out with the in vitro transcribed 3’UTR fragments of canonical A*03 (PASPD) and PASDΔ300–325, labelled with biotin, and incubated with Jurkat cell lysate. RNA bound protein was separated on PAGE and western blot was carried out using anti-Syncrip antibody.
(B) Jurkat cells were transfected with either an siRNA targeting Syncrip (Syncrip siRNA) or a control siRNA. Knockdown of Syncrip protein was confirmed after 72 hours post-transfection using western blot. (C) Seventy two hours post siRNA transfection, Jurkat cells were transfected with various HLA-A 3’UTR constructs and luciferase activity was measured after 18 hours. Inhibition of Syncrip increased luciferase activity of the constructs encoding the distal PAS (PASPD; PASD). Luciferase activity of the constructs containing only the proximal PAS (PASP) or lacking the inhibitory sequence (PASDΔ300–325) were not affected. The mean ±SE (n=6) are depicted as horizontal and vertical bars for each group, respectively, and one of three comparable experiments performed is shown. Non-parametric Wilcoxon-Mann-Whitney tests were used for statistical comparisons and two tailed p values are indicated.
