Figure 10.
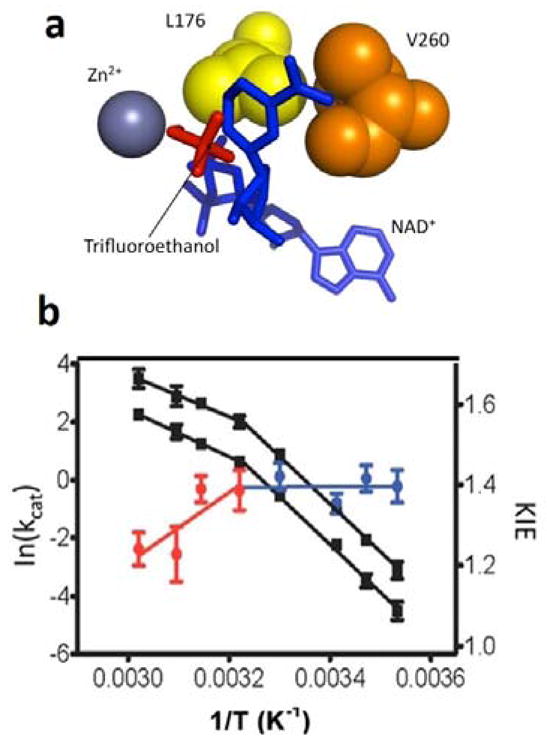
Impact of mutation at V260 in ht-ADH.74 Panel (a) displays a focused view of active-site hydrophobic side chains (Leu176 and Val260) that sit behind the nicotinamide ring of cofactor. Upon mutation of V260 to alanine (b), the break in kinetic at 30 °C in the WT ht-ADH is enhanced and the active site has been rigidified, as illustrated by the reversal of the temperature dependence of the KIE, above and below 30°C from that of WT (Figure 9a).
