Figure 12.
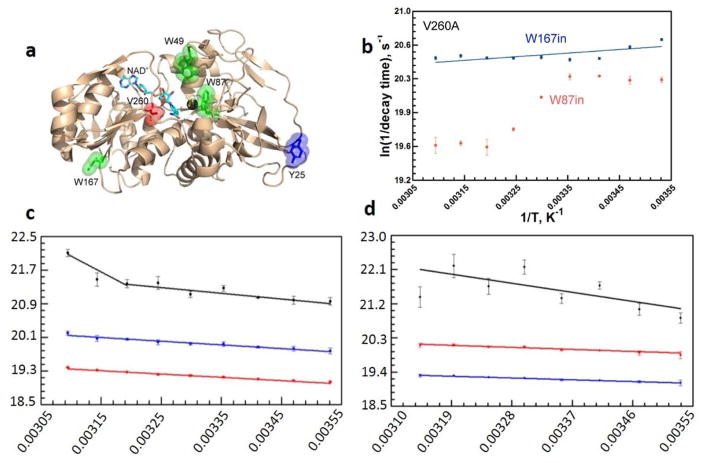
Key results from the time-resolved fluorescence studies on ht-ADH. For these studies, ht-ADH constructs were designed with one or two of the three native tryptophans substituted for phenylalanine side chains.77,78 Panel (a) shows the spatial location of these Trp residues (green sticks) with respect to the active site V260 (red) and Zn2+ (dark gray sphere). The NAD+ cofactor is represented in cyan sticks. Y25 at the subunit “Interface I” is shown for reference. Panel (b) presents the temperature dependent Stokes shifts (ps-ns timescale) that reveal two time constants, consistent with two conformations, for W87 fluorescence when the active site valine was mutated to alanine (V260A). Panels (c) and (d) show the temperature dependence of the fluorescence lifetime decay components for W87 (within the substrate binding domain) in WT and Y25A, correspondingly. A noticeable break at 30 °C is seen in the sub-nanosecond life time (black) in WT, but not for Y25A. Blue and red lines represent two longer, temperature independent components.